Preprint
Communication
Efficient Erbium-Catalyzed [3+2] Cycloaddition: Regioselective Synthesis of 1,5-Disubstituted 1,2,3-Triazoles
Altmetrics
Downloads
306
Views
258
Comments
0
supplementary.pdf (1.04MB )
Submitted:
23 November 2018
Posted:
26 November 2018
You are already at the latest version
Alerts
Abstract
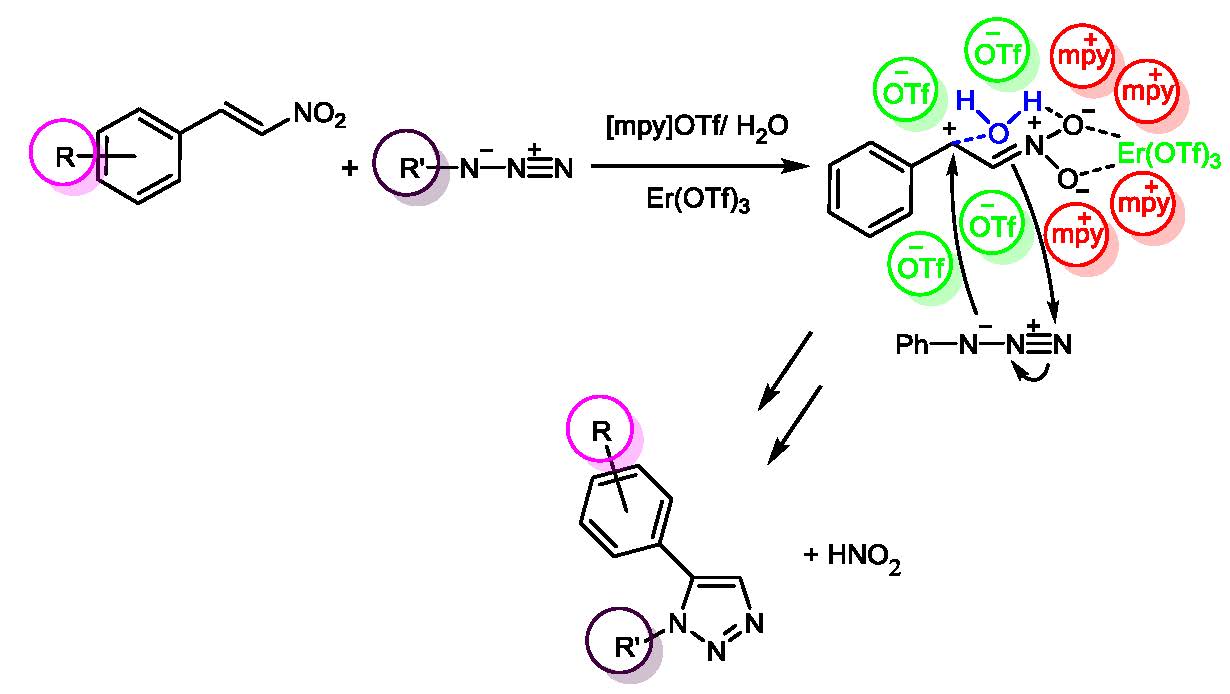
A simple procedure to obtain 1,5-disubstituted 1,2,3-triazoles, using the catalytic system erbium(III) trifluoromethanesulfonate, 1-methyl pyridinium trifluoromethanesulfonate and water is described. The reaction proceeds through an eliminative azide–olefin cycloaddition (EAOC) offering a highly regioselective approach and good yields (81–94%). The advantages of this method include simple operations of work-up and the ability of the catalytic system to be re-used five times without an evident loss in yield.
Keywords:
Subject: Chemistry and Materials Science - Organic Chemistry
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as Efficient and Reusable Homogeneous Catalyst
Antonio De Nino
et al.
,
2018
Uncatalyzed Addition and Cyclization of TSN3 to 1,1-Enediamines: An Effective Green Route to Highly Functionalized 1,2,3-Triazole Compounds
Chang-Long Yang
et al.
,
2018
Highly Efficient Synthesis of Benzimidazoles Using Microwave Irradiation
Monica Nardi
et al.
,
2022
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated












