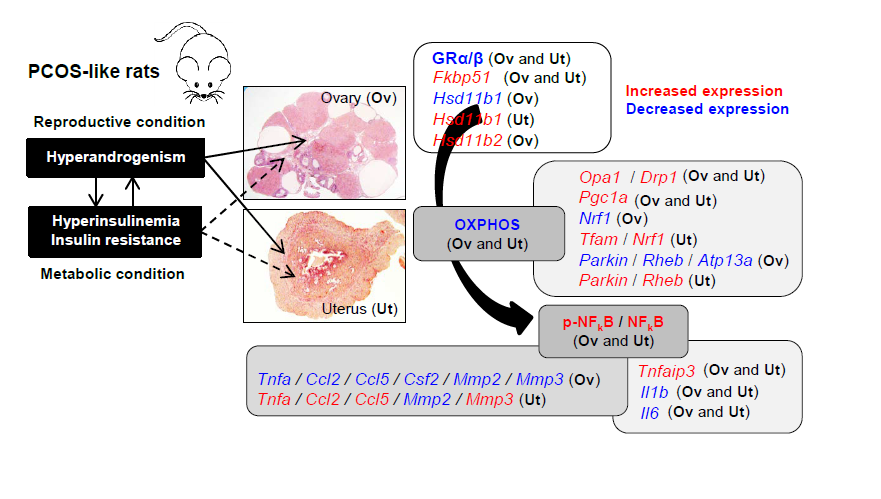
Hyperandrogenism and insulin resistance are co-pathologies of polycystic ovary syndrome (PCOS). Recent evidence has suggested that elevated local cortisol levels are associated with ovarian and endometrial insulin resistance in hyperandrogenic PCOS patients, but the molecular mechanisms underlying these clinical findings remain unclear. We and others have used chronic treatment with human chorionic gonadotropin (hCG) and insulin to create an in vivo rodent model for the onset and development of PCOS-like phenotypes. Here, we aimed to determine whether the molecular mechanisms of glucocorticoid receptor (GR) signaling, mitochondrial function, and local inflammation in the ovary and uterus are intrinsically different in PCOS-like rats compared to controls. In both the ovary and the uterus, decreased expression of two GR protein isoforms was concurrent with increased expression of Fkbp51 but not Fkbp52 mRNA in PCOS-like rats compared to controls. However, PCOS-like rats exhibited an opposite regulation of Hsd11b1 or Hsd11b2 mRNAs in the two tissues. Further, the expression of several oxidative phosphorylation-related protein components was decreased in the PCOS-like ovary and uterus, but surprisingly the expression of many genes involved in mitochondrial function and homeostasis was increased in the same tissues and animals. Additionally, PCOS-like rats showed the increased expression of ovarian and uterine NFκB signaling proteins and Tnfaip3 mRNA. In PCOS-like rats, while similar decreased expression of Il1b, Il6, and Mmp2 mRNAs was seen in the ovary and uterus, the opposite regulation of Tnfa, Ccl2, Ccl5, and Mmp3 mRNA expression was observed in the two tissues. Both ovaries and uteri from PCOS-like animals showed increased collagen deposition compared to controls. Collectively, our observations suggest that hyperandrogenism and insulin resistance disrupt ovarian and uterine GR activation and trigger compensatory or adaptive effects for mitochondrial homeostasis, allowing tissue-level maintenance of mitochondrial function in order to limit ovarian and uterine dysfunction. Our study also suggests that hyperandrogenism and insulin resistance-induced activation of NFκB signaling resulting in aberrant regulation of inflammation-related gene expression might be tissue specific in female reproductive tissues.