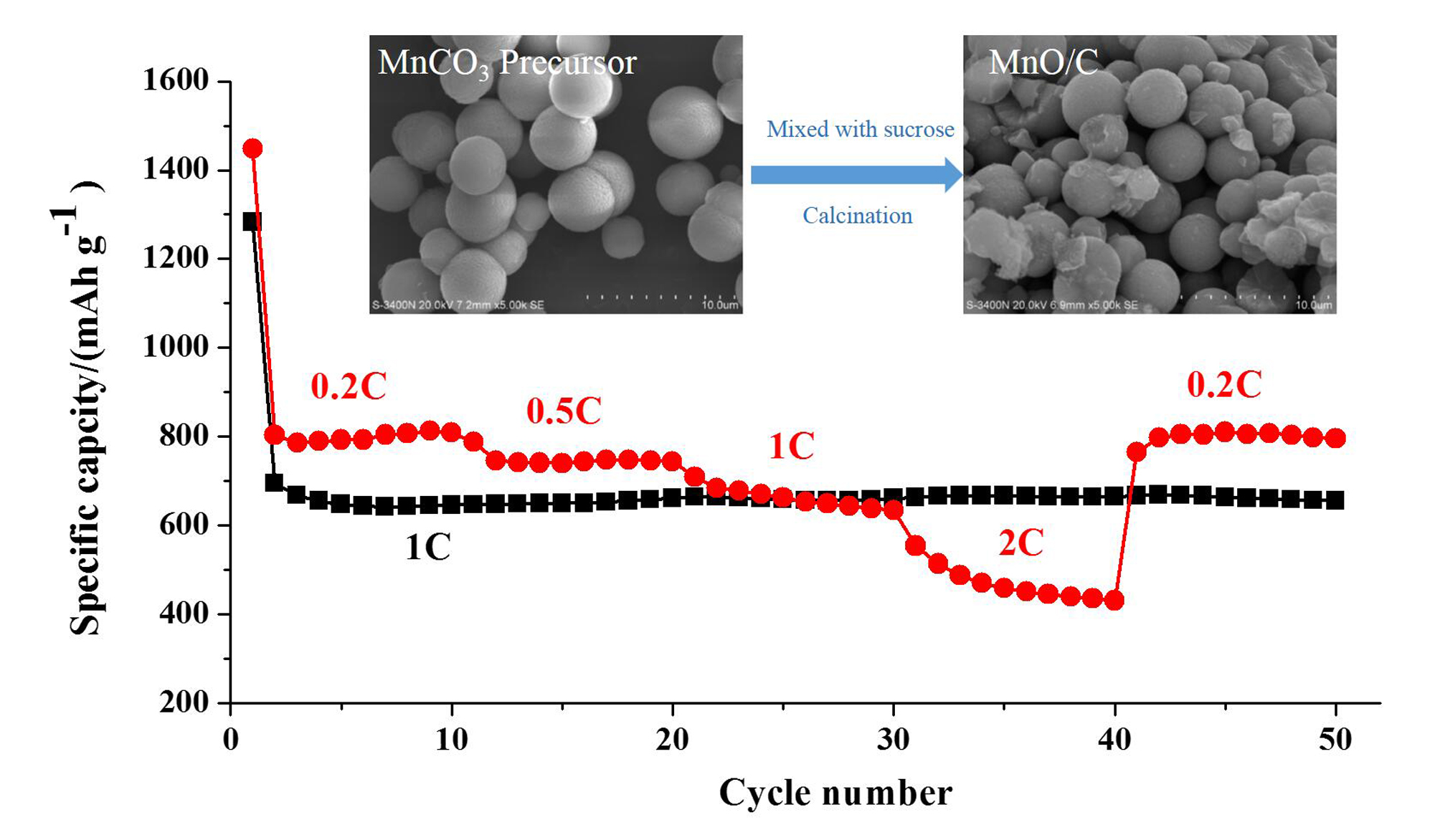
Porous MnO/C microspheres have been successfully fabricated by a fast co-precipitation method in a T-shaped microchannel reactor. The structures, compositions and electrochemical performances of the obtained MnO/C microspheres are characterized by X-ray diffraction, emission scanning electron microscopy, transmission electron microscopy (HRTEM), Brunauer–Emmett–Teller analysis, charge-discharge testing, cyclic voltammograms, and electrochemical impedance spectra. Experimental results reveal that the as-prepared MnO/C, with a specific surface area of 96.66 m2·g–1 and average pore size of 24.37 nm, exhibits excellent electrochemical performance, with a discharge capacity of 655.4 mAh·g–1 after cycling 50 times at 1 C and capacities of 808.3, 743.7, 642.6, 450.1, and 803.1 mAh·g–1 at 0.2, 0.5, 1, 2, and 0.2 C, respectively. Moreover, the controlled method of using a micro-channel reactor, which can produce larger specific surface area porous MnO/C with improved cycling performance by shortening lithium-ion diffusion distances, can be easily applied in real production on a large-scale.