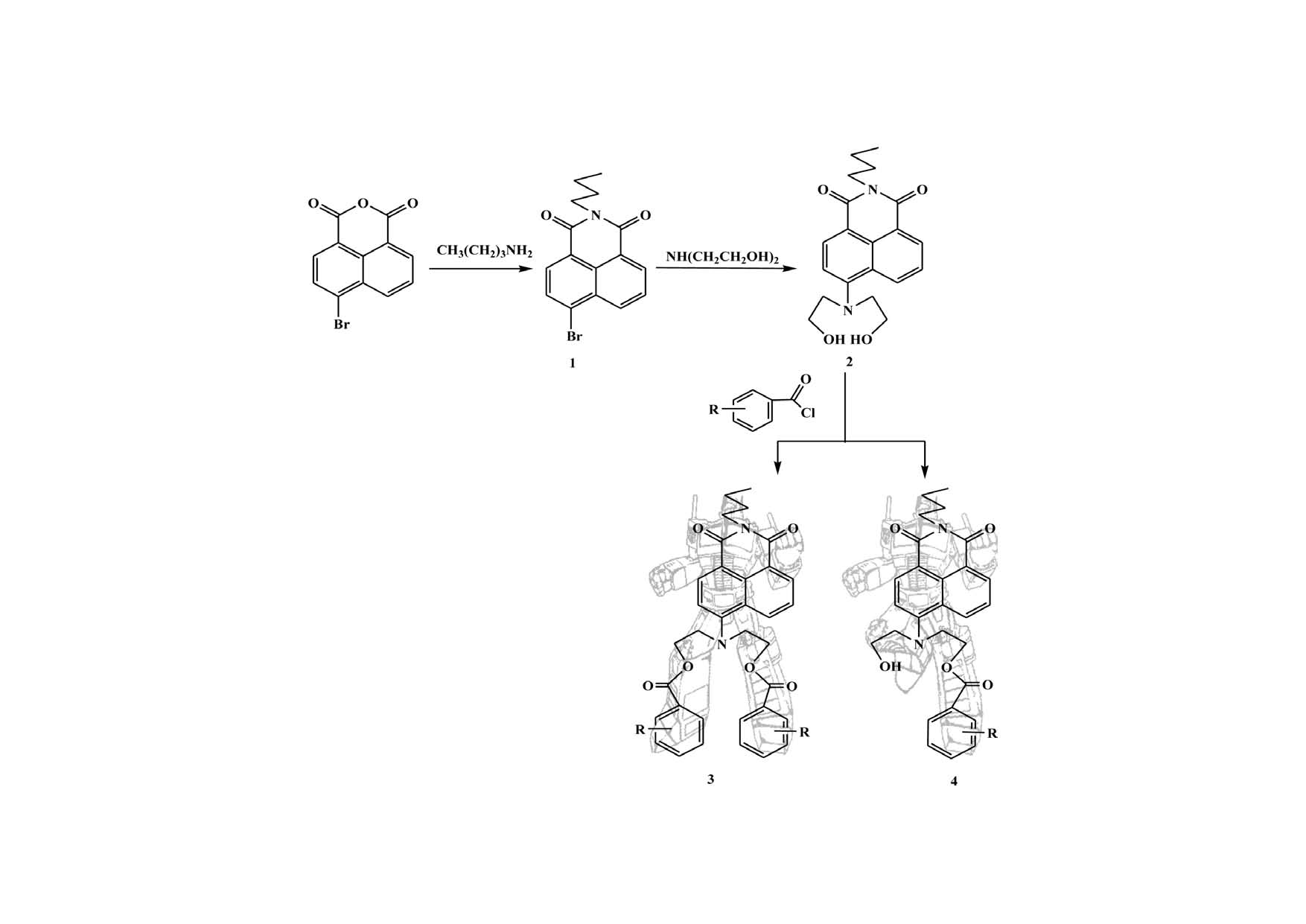
A series of novel N-n-butyl-1,8-naphthalimide derivatives were synthesized via a three-step reaction involving nucleophilic substitution and acylation. All of the compounds were characterized by IR, 1H NMR, 13C NMR, MS, and elemental analysis, and the crystal structure of N-n-butyl-4-[N’,N’-bis(2`,4`-dichlorobenzoyl)ethylamino]-1,8-naphthalimide was determined. The π-π stacking interactions and hydrogen bonds between the two molecular core planes (naphthalimide ring) and the van der Waals forces between the flexible n-butyl groups resulted in a 3D long-chain structure. The UV-vis and fluorescence properties of the title compounds were investigated. The results indicated that the monosubstituted 1,8-naphthalimide derivatives bearing an electron-donating group on the benzene ring or a structure with a larger conjugative effect exhibited enhanced fluorescence properties.