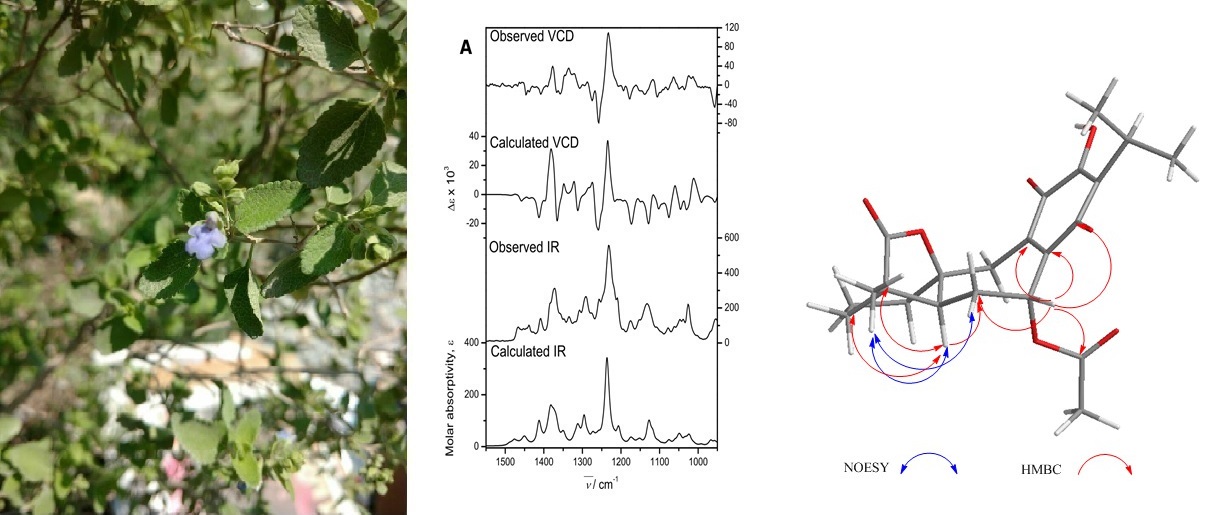
From the aerial parts of Salvia ballotiflora, eleven diterpenoids were isolated, among them, four icetexanes, and one abietane (1–5), which are first reported. Their structures were established by spectroscopic means, mainly 1H and 13C NMR, including 1D and 2D homo- and hetero-nuclear experiments. Most of the isolated diterpenoids were tested for their antiproliferative, anti-inflammatory, and radical scavenging activities using the sulforhodamine B assay on six cancer cell lines, the TPA induced ear edema test in mice, and the reduction of the DPPH assay, respectively. Some diterpenoids showed anti-proliferative activity, these being icetexanes 6 and 3, which were the most active with IC50 (µM) = 0.27 ± 0.08 and 1.40 ± 0.03, respectively, for U251 (human glioblastoma) and IC50 (µM) = 0.0.46 ± 0.05, and 0.82 ± 0.06 for SKLU-1 (human lung adenocarcinoma), when compared with adriamycin (IC50 (µM) = 0.08 ± 0.003, and 0.05 ± 0.003, as the positive control, respectively. Compounds 3 and 10 showed significant reduction of the induced ear edema of 37.4 ± 2.8 and 25.4 ± 3.0% (at 1.0 µmol/ear), respectively. Compound 4 was the sole active diterpenoid in the antioxidant assay (IC50 = 98. 4 ± 3.3), using α-tocopherol as positive control, IC50 (µM) = 31.7 ± 1.04. The diterpenoid profile found is of chemotaxonomic relevance and reinforces the evolutionary link of S. ballotiflora with other members of the section Tomentellae.