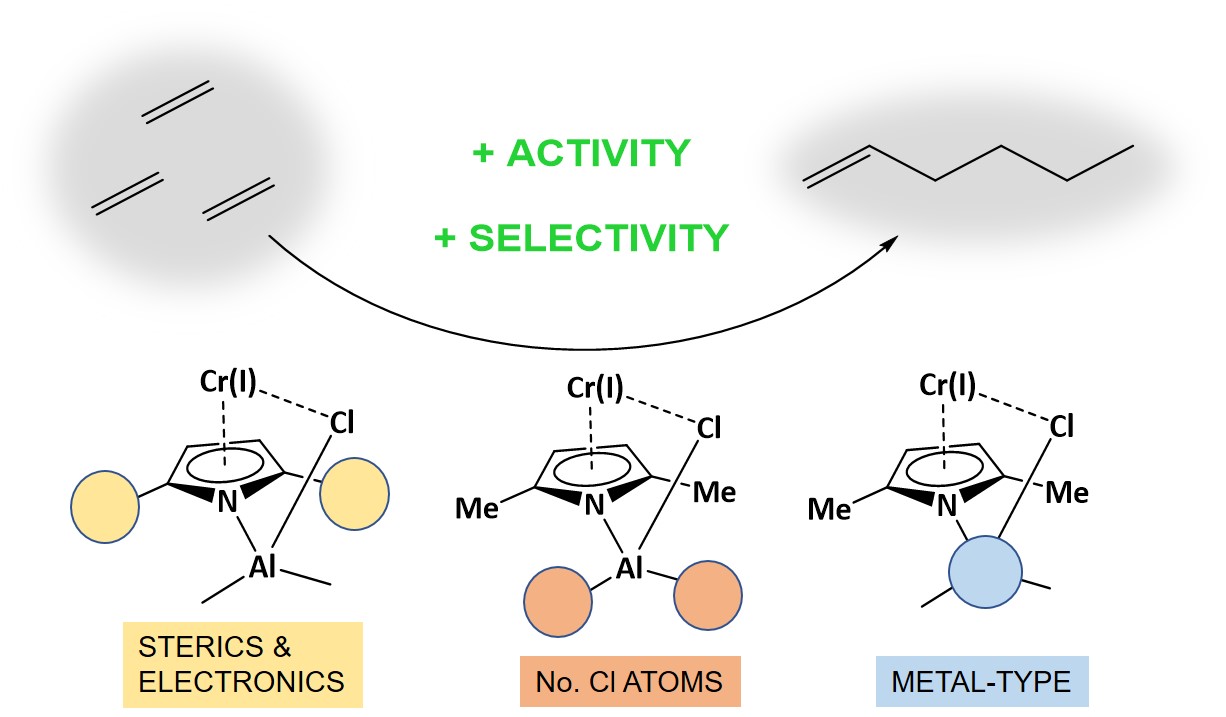
In the present work, effect of basic components on the energy pathway of ethylene oligomerization by landmark Chevron-Phillips catalyst has been explored in detail using density functional theory (DFT). Studied factors were chosen considering the main components of Chevron-Phillips catalyst, i.e. ligand, cocatalyst and halocarbon compounds, comprising i) the type of alkyl substituents in pyrrole ligand as methyl, iso-propyl, tert-butyl, and phenyl, as well as the simple hydrogen, and the electronwithdrawing fluoro and trifluoromethyl; ii) the number of Cl atoms in Al-compound (as AlMe2Cl, AlMeCl2 and AlCl3) which indicates halocarbon amount and iii) cocatalyst type as alkylboron, alkylaluminium, or alkylgallium. Besides main ingredients, solvent effect, from toluene or methylcyclohexane, on oligomerization pathway was explored as well. In this regard, the full catalytic cycles for the main product (1-hexene) formation as well as side reactions, i.e. 1-butene release and chromacyclononane formation, were calculated on the basis of the metallacycle based mechanism. Based on results, a modification on the Chevron-Phillips catalyst system, to reach higher 1-hexene selectivity and activity, is suggested.