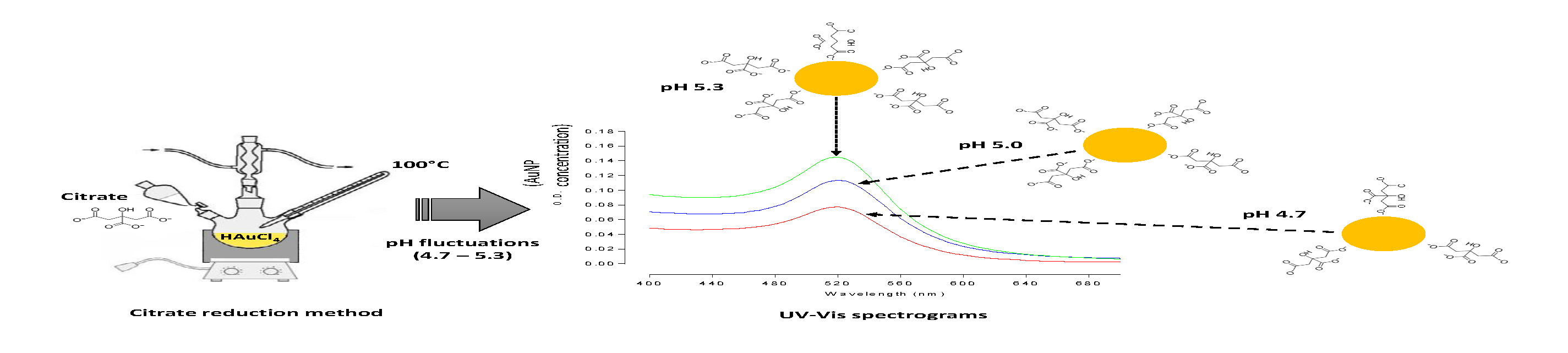
Gold nanoparticles (AuNPs) are currently under intense investigation for biomedical and biotechnology applications, thanks to their ease in preparation, stability, biocompatibility, multiple surface functionalities and size-dependent optical properties. The most commonly used method for AuNPs synthesis in aqueous solution is the reduction of tetrachloroauric acid (HAuCl4) with trisodium citrate. We observed variations in the pH and concentration of the gold colloidal suspension synthesized under standard conditions, verifying a reduction in the reaction yield by around 46% from pH 5.3 (2.4 nM) to pH 4.7 (1.29 nM). Citrate-capped AuNPs were characterized by UV-visible spectroscopy, TEM, EDS and zeta-potential measurements, revealing a linear correlation between pH and the concentration of the generated AuNPs. This result can be attributed to the adverse effect of protons both on citrate oxidation and on citrate adsorption onto the gold surface, which is required to form the stabilization layer. Overall, this study provides insight into the effect of the pH over the synthesis performance of the method, which would be of particular interest from the point of view of large-scale manufacturing processes.