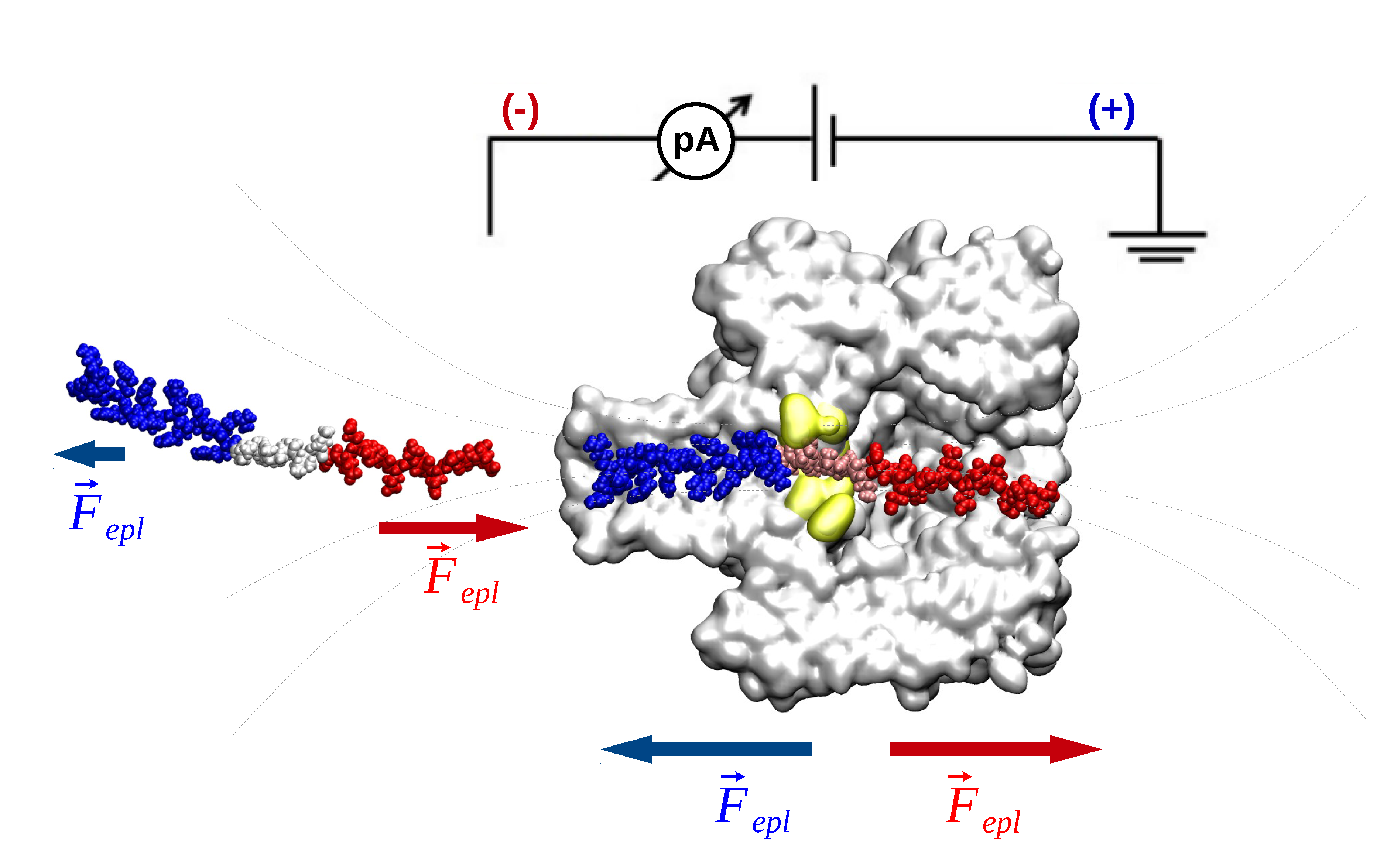
In this work we demonstrate the proof-of-concept of real-time discrimination between patches of serine or isoleucine monomers in the primary structure of custom-engineered, macro-dipole-like peptides, at uni-molecular level. We employed single-molecule recordings to examine the ionic current through the α-hemolysin (α-HL) nanopore, when hydrophilic serine or hydrophobic isoleucine residues, flanked by segments of oppositely charged arginine and glutamic amino acids functioning as a voltage-dependent ‘molecular brake’ on the peptide, were driven at controllable rates across the nanopore. The observed differences in the ionic currents blockades through the nanopore, visible at time resolutions corresponding to peptide threading through the α-HL’s constriction region, was explained by a simple model of the volumes of electrolyte excluded by either amino acid species, as groups of three serine or isoleucine monomers transiently occupy the α-HL. To provide insights into the conditions ensuring optimal throughput of peptide readout through the nanopore, we probed the sidedness-dependence of peptide association to and dissociation from the electrically and geometrically asymmetric α-HL.