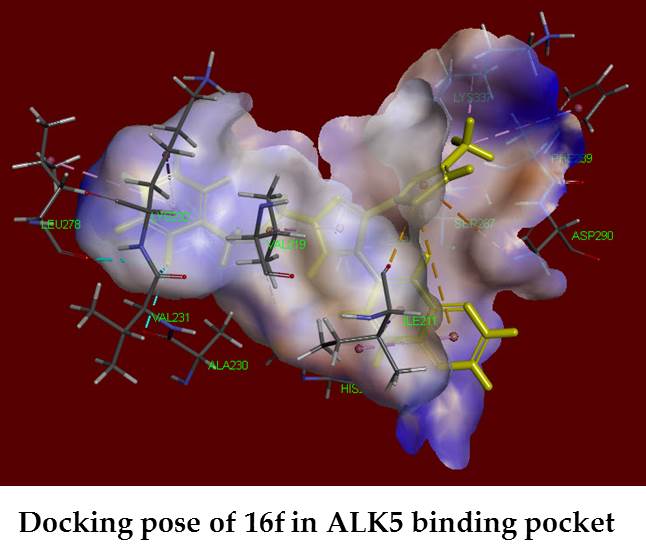
The transforming growth factor-β (TGF-β), in which overexpression have been associated with various diseases, has become an attractive molecular target for the treatment of cancers. Three series of 3-substituted-4-(quinoxalin-6-yl) pyrazoles 14a–h, 15a–h, 16a–h, 22a, 22b, 22d, 23a, 23b, 23d, 24b, and 24d were synthesized and evaluated for their activin receptor-like kinase 5 (ALK5) and p38α mitogen activated protein (MAP) kinase inhibitory activity in an enzymatic assays. Among these compounds, the most active compound 16f inhibited ALK5 phosphorylation with an IC50 value of 0.28 µM, with 98% inhibition at 10 µM. Compound 16f also had good selectivity index of >35 against p38α MAP kinase, with 9.0-fold more selective than clinical candidate, compound 3 (LY-2157299). Molecular docking study was performed to identify the mechanism of action of the synthesized compounds and their good binding interactions were observed. ADMET prediction of good active compounds showed that these ones possess good pharmacokinetics and drug-likeness behavior.