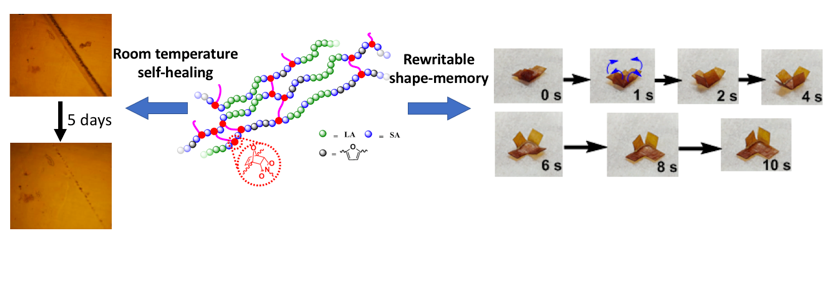
Here, we present a series of novel block copolymers (BCP) from bio-derived monomers, poly(lactic acid)-block-poly(2,5-furandimethylene succinate) (PLA-b-PFS), in which the furan groups from PFS block can be crosslinked with bis(maleimido) triethylene glycol (M2) through a Diels-Alder reaction. This dynamic crosslinking reaction leads to a network structure for enhancing the mechanical properties compared to their linear BCP analogous. Decreasing the crosslinking density leads to a decrease of glass transition temperature of BCPs and a transition from glassy to rubbery-like behavior at room temperature. This allows a wide tunablity of both elastic moduli and yields of the materials. For the lowest crosslinking density. the material exhibits an over 50% self-healing efficiency at room temperature after five days, attributed to the low Tg (15.2 C) from the introduction of PFS block, allowing sufficient chain mobility for structure re-organization. Moreover, with the appropriate selection of crosslinking density (PLA-b-PFS/M2 (6/1)), it also shows an excellent shape memory property with a high recovery rate of 96.3% and a fixity rate of 97.3%. The permanent shape can be rewriteable due to the reversibility of Diels-Alder reaction. With these advanced functionalities and ease in large-scale fabrication, the PLA-b-PFS/M2 shows great promises for self-healing coatings or films with shape memory properties in a wide variety of applications such as packaging materials