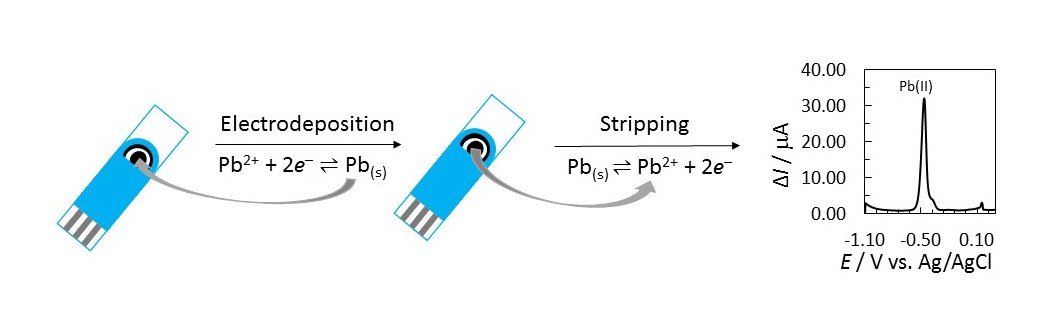
In this work, unmodified screen-printed electrode (bare SPE) and Sb-film modified SPE (SbFSPE) sensors were employed for the analysis of trace amounts of Pb(II) in non-deaerated water solutions. The modified electrode was performed in situ in 0.5 mg/L Sb(III) and 0.01 M HCl. The methodology was validated for an accumulation potential of –1.1 V vs. Ag/AgCl and an accumulation time of 60 s. A comparative analysis of bare SPE and SbFSPE showed that the detection and quantification limits decrease for the bare SPE. The method with the bare SPE showed a linear response in the 69.8–368.4 µg/L concentration range, whereas linearity for the SbFSPE was in the 24.0–319.1 µg/L concentration range. This work also reports the reason why the multiple standard addition method instead of a linear calibration curve for Pb(II) analysis should be employed. Furthermore, the analytical method employing SbFSPE was found to be more accurate and precise compared to the use of bare SPE when sensors were employed for the first time, however this performance changed significantly when these sensors were reused in the same manner. Furthermore, electrochemical impedance spectroscopy was used for the first time to analyse the electrochemical response of sensors after being used for multiple successive analyses. Surface characterisation before and after multiple successive uses of bare SPE and SbFSPE sensors, with atomic force microscopy and field emission scanning electron microscopy, showed sensor degradation. The interference effect of Cd(II), Zn(II), As(III), Fe(II), Na(I), K(I), Ca(II), Mg(II), NO3– Bi(III), Cu(II), Sn(II), and Hg(II) on the Pb(II) stripping signal was also studied. Finally, the application of SbFSPE was tested on a real water sample (from a local river), which showed high precision (RSD = 8.1%, n = 5) and accurate results.