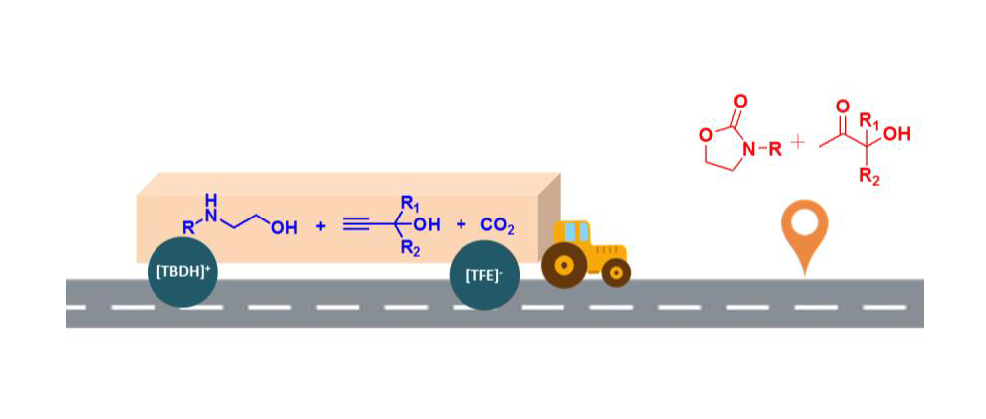
To circumvent the thermodynamic limitation of the synthesis of oxazolidinones starting from 2-aminoethanols and CO2 and realize incorporation CO2 under atmospheric pressure, a protic ionic liquid-facilitated three-component reaction of propargyl alcohols, CO2 and 2-aminoethanols was developed to produce 2-oxazolidinones along with equal amount of α-hydroxyl ketones. The ionic liquid structure, reaction temperature and reaction time were in detail investigated. And 15 mol% [TBDH][TFE] (1,5,7-triazabicylo[4.4.0]dec-5-ene trifluoroethanol) was found to be able to synergistically activate the substrate and CO2, thus catalyzing this cascade reaction under atmospheric CO2 pressure. By employing this task-specific ionic liquid as sustainable catalyst, 2-aminoethanols with different substituents were successfully transformed to 2-oxazolidinones with moderate to excellent yield after 12 h at 80 oC. This three-component reaction running under atmospheric pressure proves to be a clever detour to avoid the thermodynamic issue in the synthesis of 2-oxazolidinones starting from 2-aminoethanols and CO2.