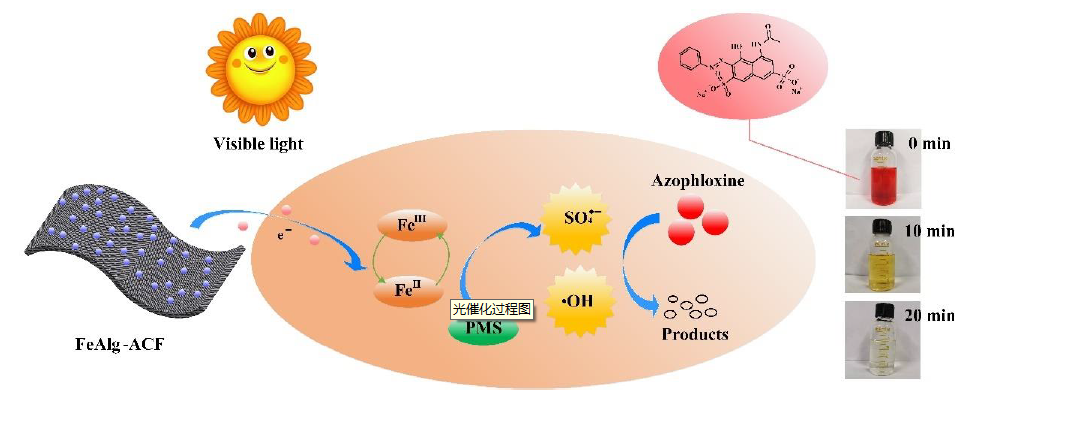
Azo dyes are the most widely used synthetic dyes in the printing and dyeing process. However, the discharge of untreated azo dyes poses potential threat for human health and aqueous ecosystem. Herein, we fabricated a novel heterogenous catalyst - activated carbon fiber-supported ferric alginate (FeAlg-ACF) . Together with peroxymonosulfate (PMS) and visible light, this photocatalytic oxidation system was used to remove an azo dye - azophloxine. The results indicated that the proposed catalytic oxidation system can remove 100% azophloxine within 24 min, while under the same system, the removal rate was only 92 % and 84 % when ferric alginate was replaced with ferric citrate and ferric oxalate respectively, which showed the superiority of activated carbon fiber-supported ferric alginate. The degradation of azophloxine is achieved by the active radicals (SO4•− and •OH) released from PMS and persistent free radicals from activated carbon fiber. After treating for 24 min, the total organic carbon of azophloxine solution (50 μmol/L) decreased from 1.82 mg/L to 79.3 μg/L and the nitrate concentration of ions increased from 0.3 mg/L to 8.6 mg/L. That is, up to 93.5% azophloxine molecules were completely degraded into inorganic compounds. Consequently, potential secondary contamination by intermediate organic products during catalytic degradation was prohibited. The azophloxine removal ratio was kept almost constant after seven cycles, indicating the recyclability and longevity of this system. Furthermore, the azophloxine removal was still promising at high concentrations of Cl-, HCO3-, CO32-. Therefore, our proposed system is potentially effective to remove dye pollutants from seawater. It provides a feasible method for the development of efficient and environmental friendly PMS activation technology combined with FeAlg-ACF, has significant academic and application value.