Submitted:
07 March 2024
Posted:
08 March 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
- Newtonian and Einsteinian gravity concepts, thus highlighting the foundations of our present understanding
- Early notions of the gravitational repulsion force and its recent revelations
1.1. Recent Revelation of the Gravitational Repulsion Force:
1.2. Challenges of some Major Assumptions in Derivation of the Kinetic Theory of Gas:
- The intermolecular force in the gaseous state is zero and as such molecules exhibit no force among themselves
2. Groundbreaking Experiment on Gravitational Repulsion:
2.1. Inferences from the Above Iodine Experiment:
- 1)
- There is a repulsion force acting on iodine particles, in the direction opposite to the Earth’s gravitational attraction force.
- 2)
- The repulsion force is dependent on the thermal energy of the particle (related to the temperature T, which is a manifestation of the thermal energy Q).
- Note:
- Heat is thermal energy. According to the classical definition, thermal energy is the net potential energy of the system. The internal energy is the net potential energy + the net kinetic energy of the system. The virial theorem assumes that the average kinetic energy over time, of a stable system consisting of a given number of particles and bound by a potential force, is proportional to the average potential energy over time 8. The virial theorem also discusses some possible mechanisms 9 dominating stellar contraction due to the gravitational attraction. Our discussion, however, is grounded on a repulsive force dependent on thermal energy which is based only on its potential energy and therefore further engagement with kinetic energy is disregarded in this paper.
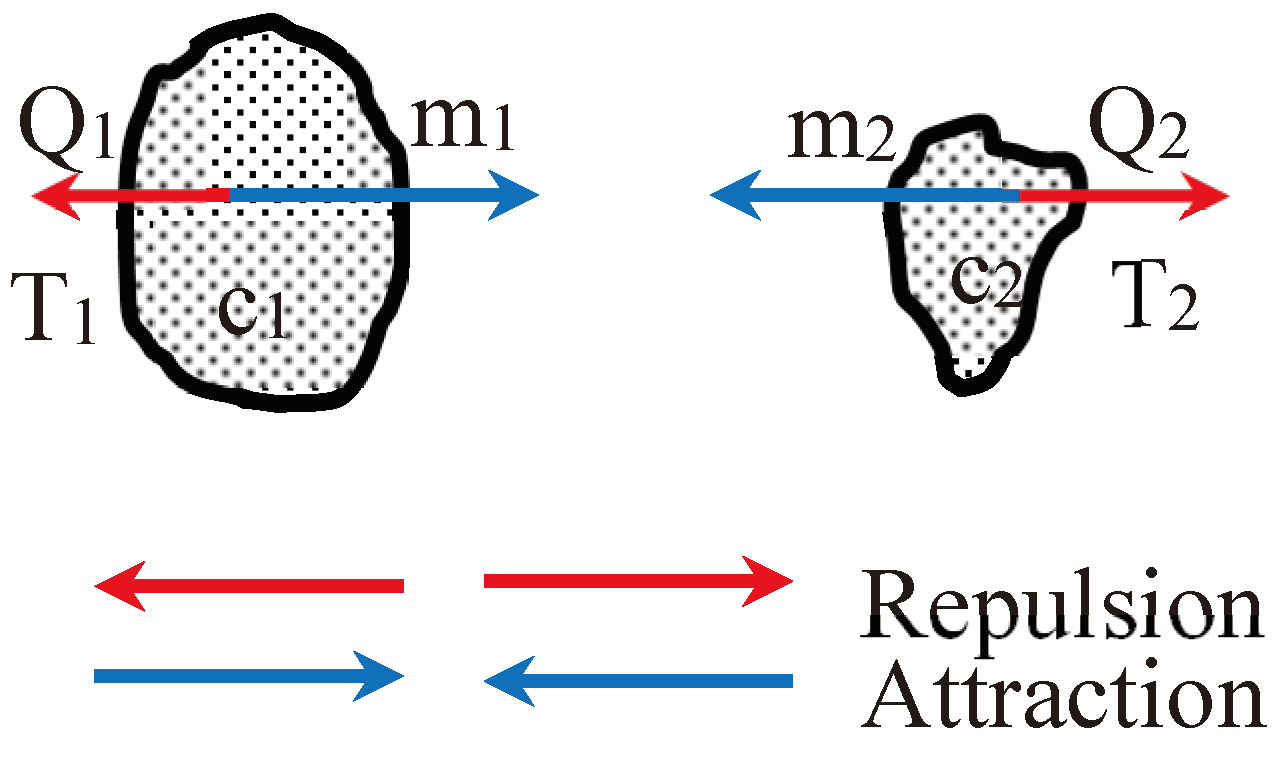
2.2. Forces between Two Entities of Matter:
- 1)
- Based on the conventional gravitational law:
- 2)
- Experimental findings presented in the Author’s publication 1b show that:
- 1)
- The model could be applied to explain certain natural phenomena observed in the atmosphere, some of which have been studied in the previous experiments in this research program [1].
- 2)
- Forces at the micro level (between gas molecules) may be generalized to the macro level to explain the behavior of the Universe.
3. Alternative Mathematical Model for the Gravitational Repulsion and Attraction Forces, Elucidating Gaseous Behavior:
3.1. Introduction to the Analysis:
- a)
- A mathematical model for the gravitational repulsion force
- b)
- A revised mathematical model for the gravitational attraction force
- c)
- Orders of magnitude of both the gravitational repulsion coefficient and the gravitational attraction coefficient
- d)
- Orders of magnitude of both gravitational repulsion and gravitational attraction forces
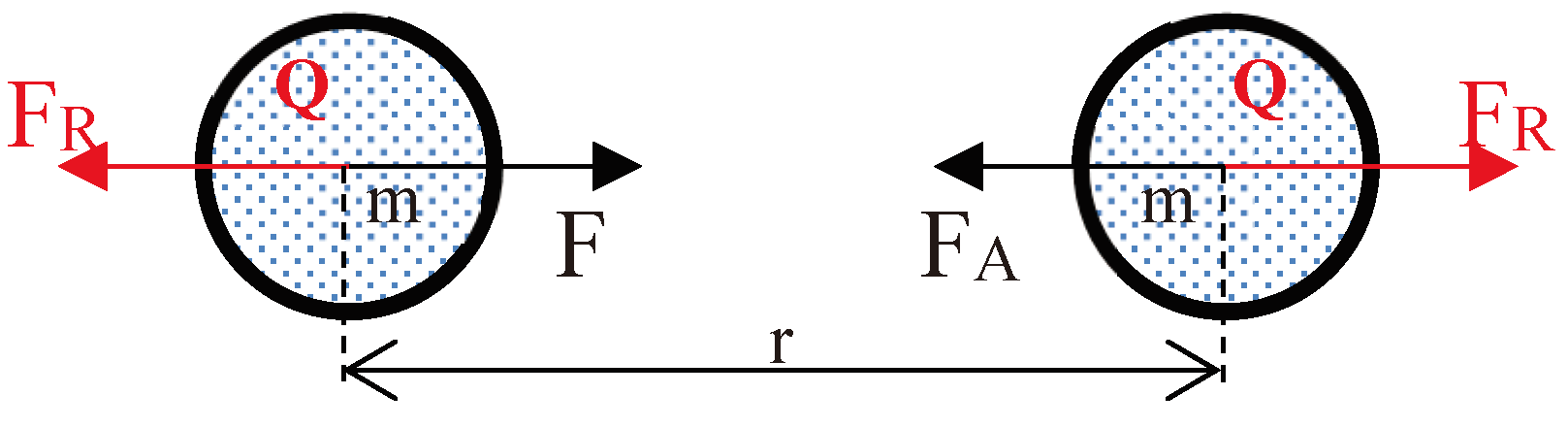
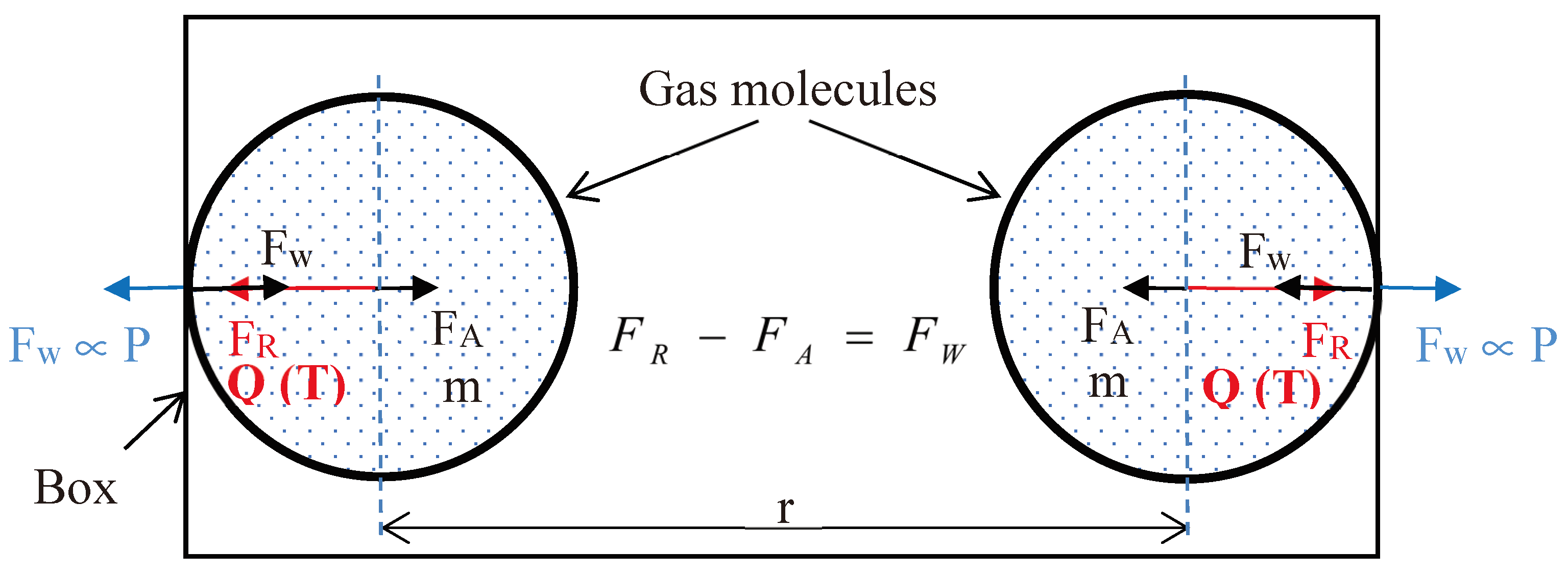
3.2. Forces between Two Gas Molecules:
3.3. Mathematical Model for Coefficient of Gravitational Repulsion and Gravitational Attraction:
- Note 1:
- c is further defined for gases 10 as: cv (Specific Heat Capacity at constant volume) and cp (Specific Heat Capacity at constant pressure).
- Note 2:
- In the classical theories, the thermal energy of a single molecule is considered as its kinetic energy (translational, vibrational, rotational, etc.), and no temperature term is associated. Nevertheless, any matter, regardless of its size, absorbs and emits energy (infrared radiation in the case of thermal energy); Stefan–Boltzmann Law 12. Gas molecule in the space is also an individual entity having its own characteristic mass and temperature, thus absorbs and emits radiation corresponding to its temperature regardless of its size 13.
3.4. Solutions for Parameters x, GR, GA, FR and FA:
4. Calculation of x, y, GR, GA, FR and FA Based on Thermodynamic Properties of Gas:
- x is a constant = 3, regardless of T and y (Figure S4 (a) in supplementary). The significance of this finding will be discussed below.
- Behaviors of GR and GA (Figures S4 (b) and (c) in supplementary) show that y = 0.5 is a significant point in this analysis.
- FR is linearly proportionate to T at any given value of y (Figure S4 (d) in supplementary information); thus, vindicating the mathematical model Equation 2. Significantly, this result confirms the findings presented in a previous paper 1b of the series of publications emanating from this research program (see Figure S6 in supplementary information). It states therein, 1b p148:
- A significant finding is that, in the linear relationship FR vs. T, the extrapolated graph intercept near the origin [≈ (0,0)] when y ≈ 0.5 (Figure S7 in supplementary information).
4.1. The Gist of the Model and the Outcomes:
5. Discussion
- extrapolation of graphs FR vs. T cross (0,0)
- negative FA tends to become positive as T approaches 0 K
- the positive value of FR increases
- the negative value of FA increases
- Heavy gas molecules (such as CFC) in the upper atmosphere
- Brownian Motion
- Condensation/evaporation/sublimation
- Expansion/contraction of gas/liquid/solid
- and more
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability
Acknowledgments
Conflicts of Interest
Availability of Data and Material
Code Availability
References
- (a) Piyadasa, C. K. G., Antigravity, an answer to nature’s phenomena including the expansion of the universe Advances of High Energy Physics, Special issue : Dark Matter and Dark Energy in General Relativity and Modified Theories of Gravity 2020, 2020 (Dark Matter and Dark Energy in General Relativity and Modified Theories of Gravity (DMDG)), 1-5; (b) Piyadasa, C. K. G., Antigravity, a major phenomenon in nature yet to be recognized. Physics Essays 2019, 32 (2), 10; (c) Piyadasa, C. K. G., Will rising water droplets change science? Canadian Journal of Pure and Applied Sciences 2012, 6 (2), 1991 - 1997; (d) Piyadasa, C. K. G., Antigravity - is it under our nose? Canadian Journal of Pure and Applied Sciences 2011, 5 (2), 1715 - 9997.
- Newton, I.; Motte, A.; Machin, J. The Mathematical Principles of Natural Philosophy; Benjamin Motte: Londonm, UK, 1729. [Google Scholar]
- (a) Villata, M., CPT symmetry and antimatter gravity in general relativity. Europhysics Letters 2011, 94 (2), 20001; (b) Wanas, M., The Other Side of Gravity and Geometry: Antigravity and Anticurvature. Advances of High Energy Physics 2012, 2012, 10; (c) Fowles, G. R., Introduction to Modern Optics. Dover Publications: New York, 1975; (d) Oikonomou, V. K.; Karagiannakis, N., Study of Antigravity in an Model and in Brans-Dicke Theory with Cosmological Constant. Journal of Gravity 2014, 2014, 625836.
- Sonntag, R.E.; Borgnakke, C.; Van Wylen, G.J. Fundamentals of Thermodynamics; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Haberle, R.M. Solar system/Sun, Atmospheres, Evolution of Atmospheres | Planetary Atmospheres: Mars; Academic Press: Oxford, 2015; pp. 168–177. [Google Scholar]
- Atreya, S. K.; Mahaffy, P. R.; Niemann, H. B.; Wong, M. H.; Owen, T. C. , Composition and origin of the atmosphere of Jupiter - an update, and implications for the extrasolar giant planets. Planetary and Space Science 2003, 51, 105. [Google Scholar] [CrossRef]
- Cambel, A.B.; Duclos, D P.; Anderson, T.P. Real Gases; Academic Press: Boston, 1963. [Google Scholar]
- Singh, A.K. Structure, Synthesis, and Application of Nanoparticles; Academic Press: Boston, 2016; pp. 19–76. [Google Scholar]
- Zloshchastiev, K. G. , Temperature-driven dynamics of quantum liquids: Logarithmic nonlinearity, phase structure and rising force. International Journal of Modern Physics B 2019, 33(17), 1950184. [Google Scholar] [CrossRef]
- Obert, E.F. Concepts of Thermodynamics; McGraw-Hill: New York, 1960. [Google Scholar]
- Keenan, J.H.; Chao, J.; Kaye, J. Gas Tables; John Wiley and sons: New York, 1980; p. 217. [Google Scholar]
- Kaiserl. Akademie der Wissenschaften in, W., Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe. Abt. 2, Mathematik, Physik, Chemie, Mechanik, Meteorologie und Astronomie. 1872, 32 v. (vol. 65-96).
- Golovkin, B. G. Physical meaning temperature of gas and separate molecule. World Scientific News 2018, 94(2), 8. [Google Scholar]
- Keenan, J. H.; Kaye, J., Gas Tables: Thermodynamic Properties of Air, Products of Combustion and Component Gases, Compressible Flow Function Including Those of Ascher H. Shapiro and Gilbert M. Edelman. J. Wiley: 1948.
- Michaud, A. , On The Magnetostatic Inverse Cube Law and Magnetic Monopoles. International Journal of Engineering Research and Development 2013, 7(5), 16. [Google Scholar]
- (a) Iorio, L. (a) Iorio, L., Effect of sun and planet-bound dark matter on planet and satellite dynamics in the solar system. Journal of Cosmology and Astroparticle Physics 2010, 2010 (05), 018-018; (b) page, E. T. f. t. a. Dark Matter May Explain the Puzzling Change in Earth-Sun Distance. https://www.technologyreview.com/2010/01/14/206662/dark-matter-may-explain-the-puzzling-change-in-earth-sun-distance/.
- Abbott, B.P.; Abbott, R.; Abbott, T.D.; Acernese, F.; Ackley, K.; Adams, C.; Adams, T.; Addesso, P.; Adhikari, R.X.; Adya, V.B.; et al. , A gravitational-wave standard siren measurement of the Hubble constant. Nature 2017, 551(7678), 85–88. [Google Scholar]
- (a) Sidharth, B. G., THE UNIVERSE OF FLUCTUATIONS. International Journal of Modern Physics A 1998, 13 (15), 2599-2612; (b) Riess, A. G.; Filippenko, A. V.; Challis, P.; Clocchiatti, A.; Diercks, A.; Garnavich, P. M.; Gilliland, R. L.; Hogan, C. J.; Jha, S.; Kirshner, R. P.; Leibundgut, B.; Phillips, M. M.; Reiss, D.; Schmidt, B. P.; Schommer, R. A.; Smith, R. C.; Spyromilio, J.; Stubbs, C.; Suntzeff, N. B.; Tonry, J., Observational Evidence from Supernovae for an Accelerating Universe and a Cosmological Constant. The Astronomical Journal 1998, 116 (3), 1009-1038; (c) Perlmutter, S.; Aldering, G.; Valle, M. D.; Deustua, S.; Ellis, R. S.; Fabbro, S.; Fruchter, A.; Goldhaber, G.; Groom, D. E.; Hook, I. M.; Kim, A. G.; Kim, M. Y.; Knop, R. A.; Lidman, C.; McMahon, R. G.; Nugent, P.; Pain, R.; Panagia, N.; Pennypacker, C. R.; Ruiz-Lapuente, P.; Schaefer, B.; Walton, N., Discovery of a supernova explosion at half the age of the Universe. Nature 1998, 391 (6662), 51-54; (d) Lima, J. A. S., Alternative dark energy models: an overview. Brazilian Journal of Physics 2004, 34, 194-200.
- Piyadasa, C. K. G., An alternative model of gravitational forces in nature using the combined effects of repulsion and attraction forces on gaseous molecules. PREPRINT (Version 1) available at Research Square 2021, (rs-1173458).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




