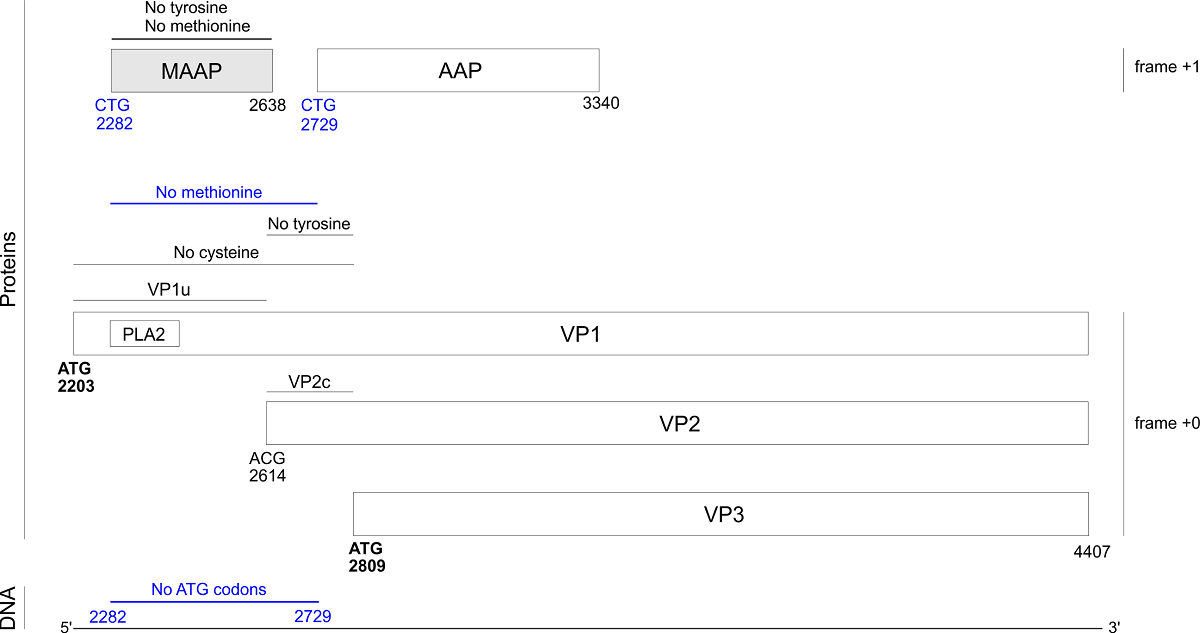
Adeno-associated viruses (AAVs, genus dependoparvovirus) are promising gene therapy vectors. In strains AAV1-12, the capsid gene VP1 encodes a recently discovered protein, MAAP, in an overlapping frame. MAAP binds the cell membrane by an unknown mechanism. We discovered that MAAP is also encoded in bovine AAV and in porcine AAVs (which have shown promise for gene transfer into muscle tissues), in which it is probably translated from a non-canonical start codon. MAAP is predicted to be mostly disordered except for a predicted C-terminal, membrane-binding amphipathic α-helix. MAAP has a highly unusual composition. In particular, it lacks internal methionines, and is devoid of tyrosines in most strains. Unexpectedly, we discovered that the N-terminus of VP1 also lacks several amino acids. In all AAVs that encode MAAP, the first 200 aas of VP1 are devoid of internal methionines, probably owing to a selection against ATG codons that could prevent translation of MAAP and of capsid isoforms (VP2, VP3). The N-terminus of VP1 also lacks cysteines, likely to avoid the formation of disulfide bridges when it becomes exposed outside of the capsid during post-endocytic trafficking. Finally, the region common to VP1 and VP2 lacks tyrosine in the vast majority of AAVs that encode MAAP. Avoiding these "forbidden" aas in MAAP and VP1 when creating recombinant AAV capsids might increase the efficiency of capsid design. Conversely, the presence of "forbidden" aas in some rare strains probably indicates that they have unusual properties that could help us understand the viral cycle.