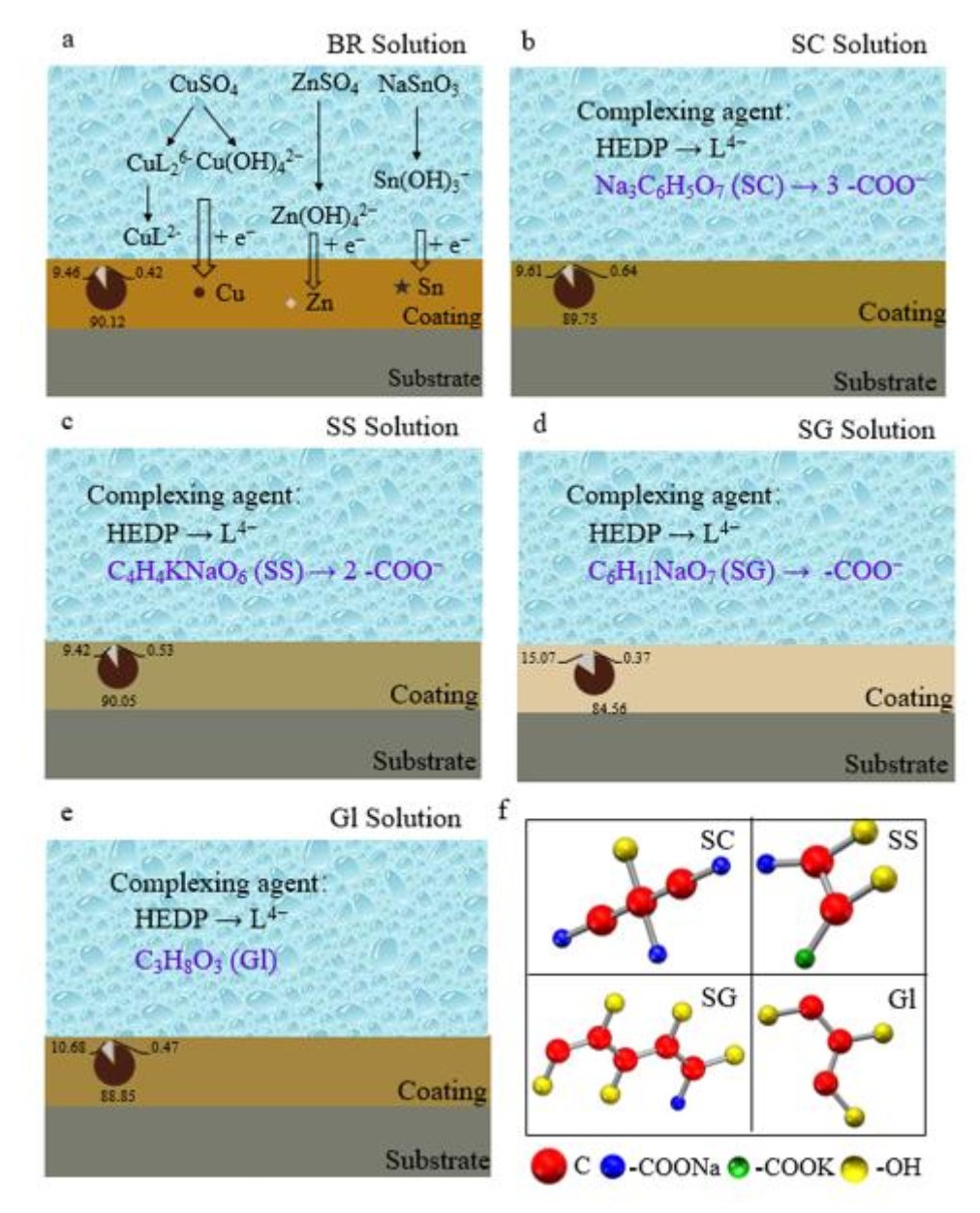
The requirements for using noncyanide imitation gold plating as decorative electroplating are increasing; thus, continuously improving the quality of the coating of the imitation gold plating and optimizing the coating process have become the current priority. In this work, hydroxyethylidene diphosphonic acid (HEDP) was used as the main complexing agent, CuSO4·5H2O, ZnSO4·7H2O and NaSnO3·3H2O were the main salts, and NaOH and anhydrous sodium carbonate were used as the buffers to prepare the electroplating solution. Using sodium citrate (SC), sodium potassium tartrate (SS), sodium gluconate (SG), and glycerol (Gl) as four additives, the effects of the number of carboxyl groups on the properties of a Cu-Zn-Sn alloy coating were compared. The electrochemical analysis showed that Cu-Zn-Sn alloy codeposition occurred at -0.50 V vs. Hg|HgO. The scanning electron microscopy (SEM) results showed that the particle size of the coatings obtained with carboxyl-containing additives was more uniform than that obtained with the electroplating solution without additives. The X-ray fluorescence spectrometry (XRF) analysis revealed that the composition of the Cu-Zn-Sn alloy coating obtained by using SC as an additive in the electroplating solution was 89.75 wt% Cu, 9.61 wt% Zn, and 0.64 wt% Sn, and the color of the coating was golden yellow. The X-ray diffraction (XRD) pattern showed that the coating was a mixture of Cu, Cu5Zn8, CuSn, Cu6Sn5, and CuZn phases. The analysis of the electroplating solution by UV, IR and NMR spectroscopy methods indicates that the additives improve the coating by affecting the complexation reaction of metal ions. These results can provide technical and theoretical guidance for developing Cu-Zn-Sn ternary alloy electrodeposition technology with the new cyanide-free HEDP alkaline electroplating system.