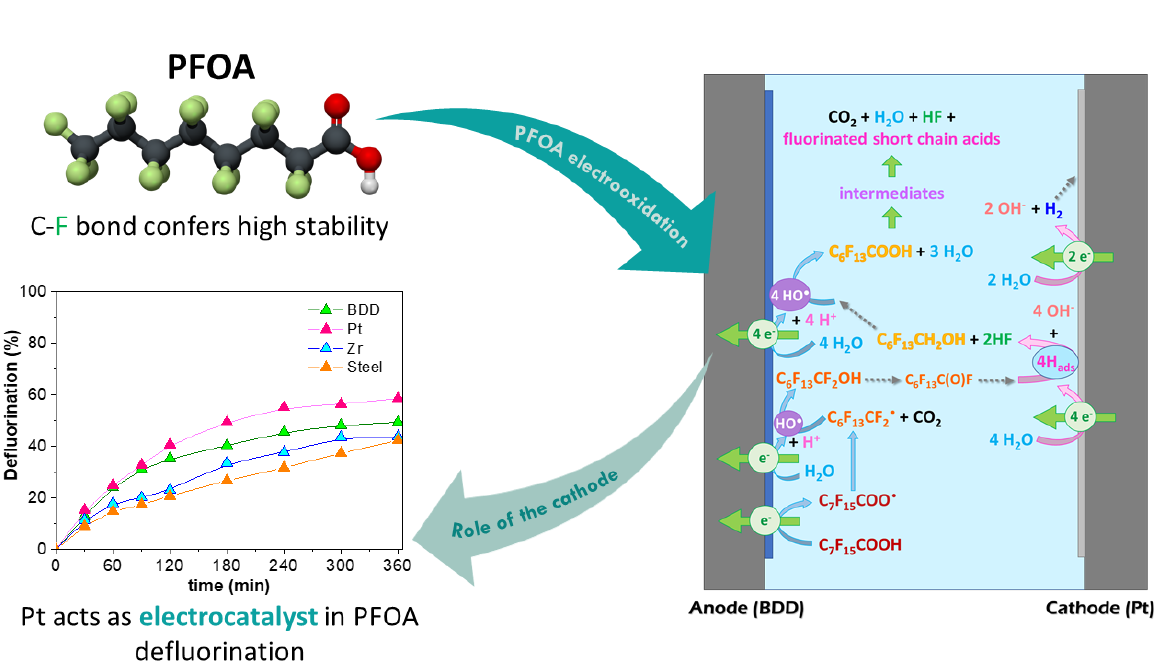
Perfluorooctanoic acid (PFOA), C7F15COOH, has been widely employed over the past fifty years, causing an environmental problem due to its dispersion and low biodegradability. Furthermore, the high stability of this molecule, conferred by the high strength of the C-F bond makes it very difficult to remove. In this work, electrochemical techniques are applied for PFOA degradation in view to study the influence of the cathode on defluorination. For this purpose, boron doped diamond (BDD), Pt, Zr and stainless steel have been tested as cathodes working with BDD anode at low electrolyte concentration (3.5 mM) to degrade PFOA at 100 mg/L. Among these cathodic materials, Pt improves the defluorination reaction. The electro-degradation of a PFOA molecule starts by a direct exchange of one electron at the anode and then follows a complex mechanism involving reaction with hydroxyl radicals and adsorbed hydrogen on the cathode. It is assumed that Pt acts as an electrocatalyst, enhancing PFOA defluorination by the reduction reaction of perfluorinated carbonyl intermediates on the cathode. The defluorinated intermediates are then more easily oxidized by HO• radicals. Hence, high mineralization (xTOC: 76.1%) and defluorination degrees (xF-: 58.6%) were reached with Pt working at current density j = 7.9 mA/cm2. This BDD-Pt system reaches a higher efficiency in terms of defluorination for a given electrical charge than previous works reported in literature. Influence of the electrolyte composition and initial pH are also explored.