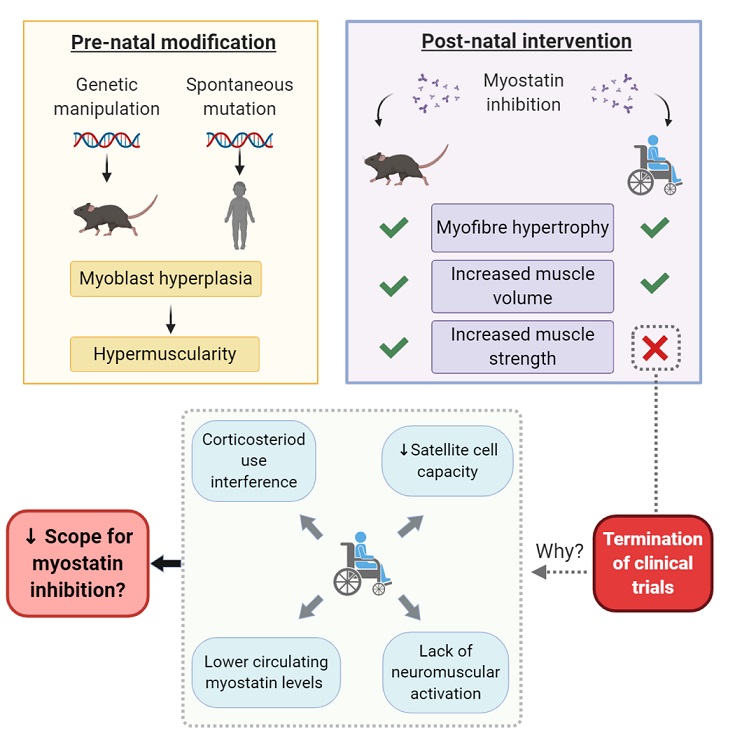
Myostatin inhibition therapy has held much promise for the treatment of muscle wasting disorders. This is particularly true for the fatal myopathy, Duchenne Muscular Dystrophy (DMD). Following on from promising pre-clinical data in dystrophin-deficient mice and dogs, several clinical trials were initiated in DMD patients using different modality myostatin inhibition therapies. All failed to show modification of disease course as dictated by the primary and secondary outcomes measures selected: the myostatin inhibition story thus far, is a failed clinical story. These trials have recently been extensively reviewed and reasons why pre-clinical data collected in animal models has failed to translate into clinical benefit to patients has been purported. However, the biological mechanisms underlying translational failure need to be examined to ensure future myostatin inhibitor development endeavors do not meet with the same fate. Here, we explore the biology which could explain the failed translation of myostatin inhibitors in the treatment of DMD.