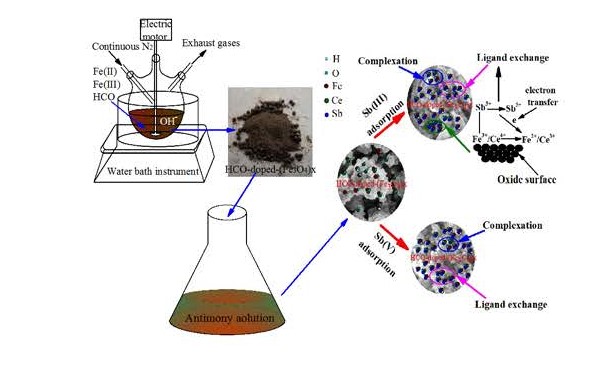
Concern over potential antimony mediated toxicity from mining and smelting activities has instigated novel concepts toward removing aqueous antimony ions. The iron based adsorbent Fe3O4/HCO was found to be efficient for treating antimony-containing wastewater However, ineffective methodology for preparation limited its effective adsorption capacity and thus wider application. In this study, a new type of HCO-doped-(Fe3O4)x adsorbent was prepared by co-precipitation method for doping Fe3O4 into HCO sludge (HCO), thereby improving adsorption performance for Sb(III) and Sb(V) ions, with the maximum adsorbing capacity being 44.46 mg/g and 47.91 mg/g, respectively. According to the results of BET, SEM-EDS, XRD and XPS, it were confirmed that the FeOOH and X≡Fe-OH were formed during the preparation process, bring about the increased the surface area, thus resulting in further increase of surface area, hydroxyl groups and the net negative ionic charge. Moreover, the adsorption kinetics followed the pseudo-second-order kinetic model which indicated that adsorption process of Sb(III)/Sb(V) by HCO-doped-(Fe3O4)x adsorbent was controlled by chemical reaction. The main adsorption mechanism is that antimony ion and amorphous iron oxide X≡Fe-OH undergo coordination exchange reaction and complexation reaction with CeO2 or Ce2O3. Furthermore, HCO-doped-(Fe3O4)x could adapt to wide pH and had stable adsorption ability after regeneration. The good adsorption performance of HCO-doped-(Fe3O4)x makes it a potential applications of adsorbent for removal of antimony.