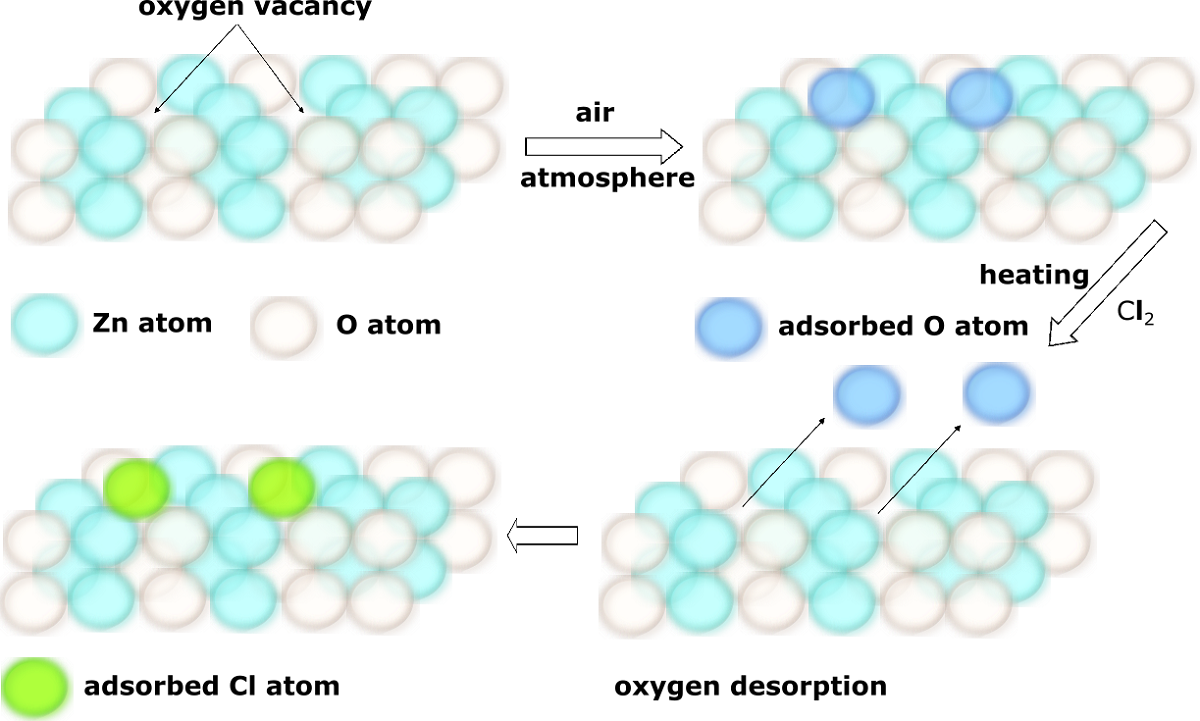
In the article we present the results concerning the impact of structural and chemical properties of zinc oxide in various morphological forms, on its gas-sensitive properties tested in an atmosphere containing a very aggressive gas such as chlorine. Two types of ZnO sensor layers obtained by two different technological methods were used. Their morphology, crystal structure, specific surface area, porosity, surface chemistry and structural defects were characterized, and then compared with gas-sensitive properties in a chlorine-containing atmosphere. To achieve this goal scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and photoluminescence spectroscopy (PL) methods were used. The sensing properties of obtained active layers were tested by temperature stimulated conductance method (TSC). We have noticed that their response in chlorine atmosphere is not determined by the size of the specific surface or porosity. The obtained results showed that the structural defects of ZnO crystals play the most important role in chlorine detection. We demonstrated that the Cl2 adsorption is a concurrent process to oxygen adsorption. Both of them occur on the same active species (oxygen vacancies). They concentration is higher on the side planes of the zinc oxide crystal than the others. Thanks to the conducted studies authors demonstrated that to develop a new gas sensor devices not only changing of active layer chemical composition but also controlling its crystal structure and morphology could be used.