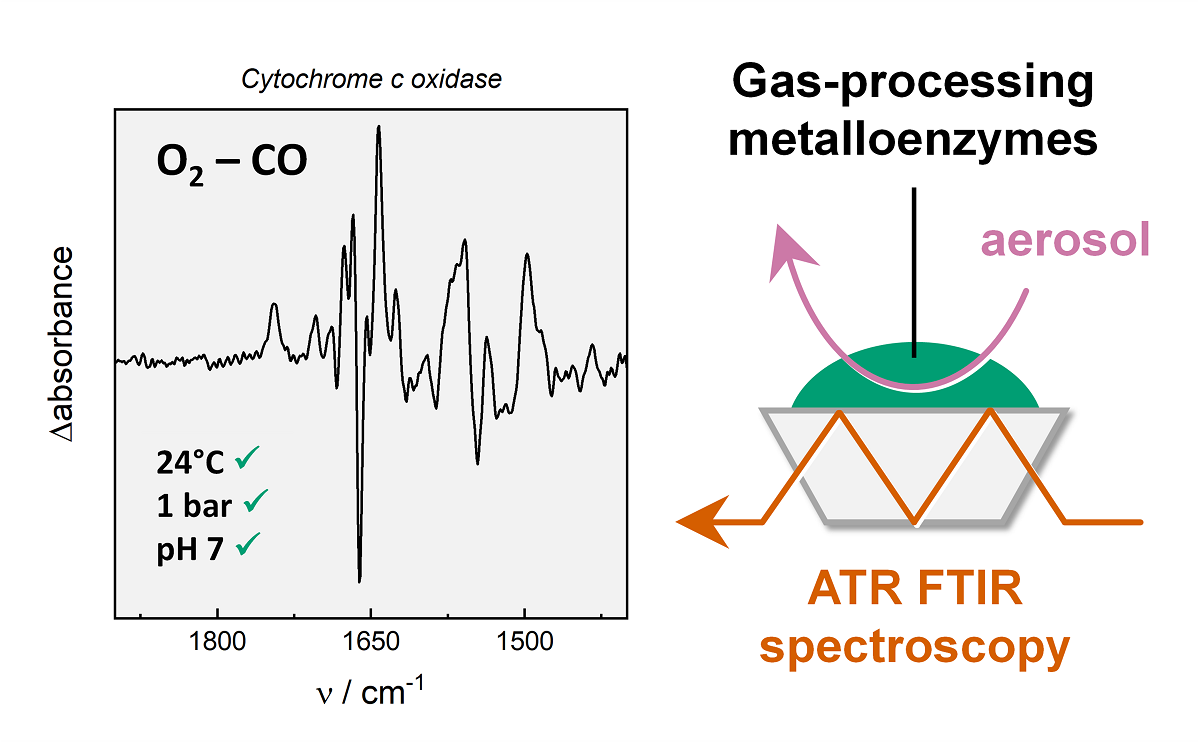
Earth-abundant transition metals like iron, nickel, copper, molybdenum, and vanadium have been identified as essential constituents of the cellular gas metabolism in all kingdoms of life. Associated with biological macromolecules, gas-processing metalloenzymes (GPMs) are formed that catalyse a variety of redox reactions. This includes the reduction of O2 to water by cytochrome c oxidase (‘complex IV’), the reduction of N2 to NH4 by nitrogenase, as well as the reduction of protons to H2 (and oxidation of the later) by hydrogenase. GPMs perform at ambient temperature and pressure, in the presence of water, and often extremely low educt concentrations, thus serving as natural examples for efficient catalysis. Facilitating the design of biomimetic catalysts, biophysicist thrive to understand the reaction principles of GPMs making use of various techniques. In this perspective, I will introduce Fourier-transform infrared spectroscopy in attenuated total reflection configuration (ATR FTIR) for the analysis of GPMs like cytochrome c oxidase, nitrogenase, and hydrogenase. Infrared spectroscopy provides information about the geometry and redox state of the catalytic cofactors, the protonation state of amino acid residues, the hydrogen-bonding network, and protein structural changes. I developed an approach to probe and trigger the reaction of GPMs by gas exchange experiments, exploring the reactivity of these enzymes with their natural reactants. This allows recording sensitive ATR FTIR difference spectra with seconds time resolution. Finally yet importantly, infrared spectroscopy is an electronically non-invasive technique that allows investigating protein samples under biologically relevant conditions, i.e., at ambient temperature and pressure, and in the presence of water.