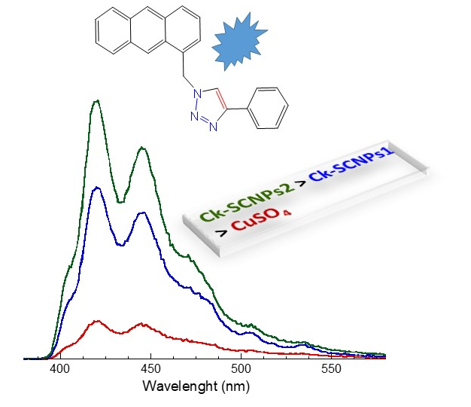
“Clickase” single-chain nanoparticles (Ck-SCNPs) are folded, enzyme-mimetic unimolecular polymeric nano-objects containing copper (Cu) ions able to catalyze the azide-alkyne Huisgen cycloaddition reaction in water and/or selected organic solvents, often in the presence of a reductant. Herein, we investigate the effect of morphology on catalytic activity of Ck-SCNPs synthesized by means of two different routes. An amphiphilic random copolymer composed of oligo(ethylene glycol) methyl ether methyl methacrylate (OEGMA) and 2-acetoacetoxy ethyl methacrylate (AEMA) units was used as precursor of these Ck-SCNPs. Folding was promoted through metal complexation between Cu(II) ions and beta-ketoester-containing AEMA moieties. The first route resulted in Ck-SCNPs1 containing Cu ions homogeneously distributed within each nanoparticle, whereas the second one promoted intra-chain clustering of Cu ions inside Ck-SCNPs2. A model fluorogenic “click” reaction between 9-(azidomethyl)anthracene and phenylacetylene, which was catalyzed either by Ck-SCNPs1 or Ck-SCNPs2, was used to unravel the effect of morphology on catalytic activity. This work paves the way to improve the catalytic activity of metallo-folded SCNPs through control of the intra-chain distribution of catalytic sites.