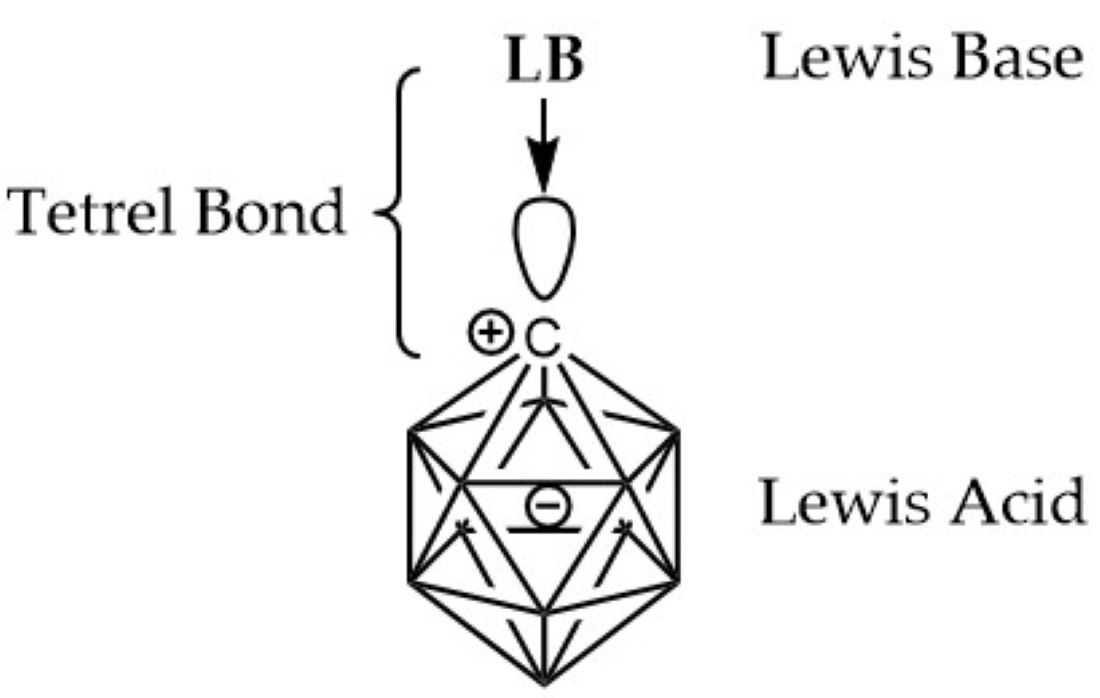
High-level quantum-chemical computations (G4MP2) are carried out in the study of complexes featuring tetrel bonding between the carbon atom in the carbenoid CB11H11 - obtained by hydride removal in the C-H bond of the known closo-monocarbadodecaborate anion CB11H12() and acting as Lewis acid (LA) - and Lewis bases (LB) of different type; the electron donor groups in the tetrel bond feature carbon, nitrogen, oxygen, fluorine, silicon, phosphorus, sulphur and chlorine atomic centers in neutral molecules as well as anions H(-), OH(-) and F(-). The empty radial 2pr vacant orbital on the carbon center in CB11H11 , which corresponds to the LUMO, acts as a Lewis acid or electron attractor, as shown by the molecular electrostatic potential (MEP) and electron localization funcion (ELF). The thermochemistry and topological analysis of the complexes {CB11H11:LB} are comprehensively analyzed, and classified according to sharing or closed-shell interactions. ELF analysis shows that the tetrel C···X bond ranges from very polarised bonds, as in H11B11C:F() to very weak interactions as in H11B11C···FH and H11B11C···O=C=O.