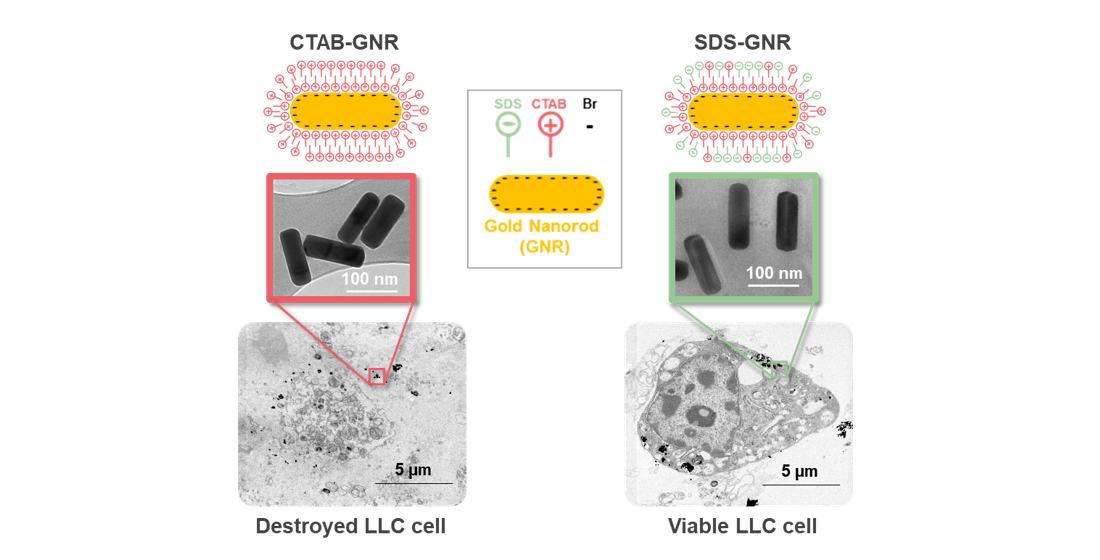
Due to their well-defined plasmonic properties, gold nanorods (GNRs) can be fabricated with optimal light absorption in the near-infrared region of the electromagnetic spectrum, which make them suitable for cancer-related theranostic applications. However, their controversial safety profile, as a result of surfactant stabilization during synthesis, limits their clinical translation. We report a facile method to improve GNR biocompatibility through the presence of sodium dodecyl sulfate (SDS). GNRs (120 x 40 nm) were synthesized through a seed-mediated approach, using cetyltrimethylammonium bromide (CTAB) as a cationic surfactant to direct the growth of nanorods and stabilize the particles. Post-synthesis, SDS was used as an exchange ligand to modify the net surface charge of the particles from positive to negative while maintaining rod stability in an aqueous environment. GNR cytotoxic effects, as well as the mechanisms of their cellular uptake, were examined in two different cancer cell lines, Lewis lung carcinoma (LLC) and HeLa cells. We not only found a significant dose-dependent effect of GNR treatment on cell viability but also a time-dependent effect of GNR surfactant charge on cytotoxicity over the two cell lines. Our results promote a better understanding of how we can mediate the undesired consequences of GNR synthesis byproducts when exposed to a living organism, which so far has limited GNR use in cancer theranostics.