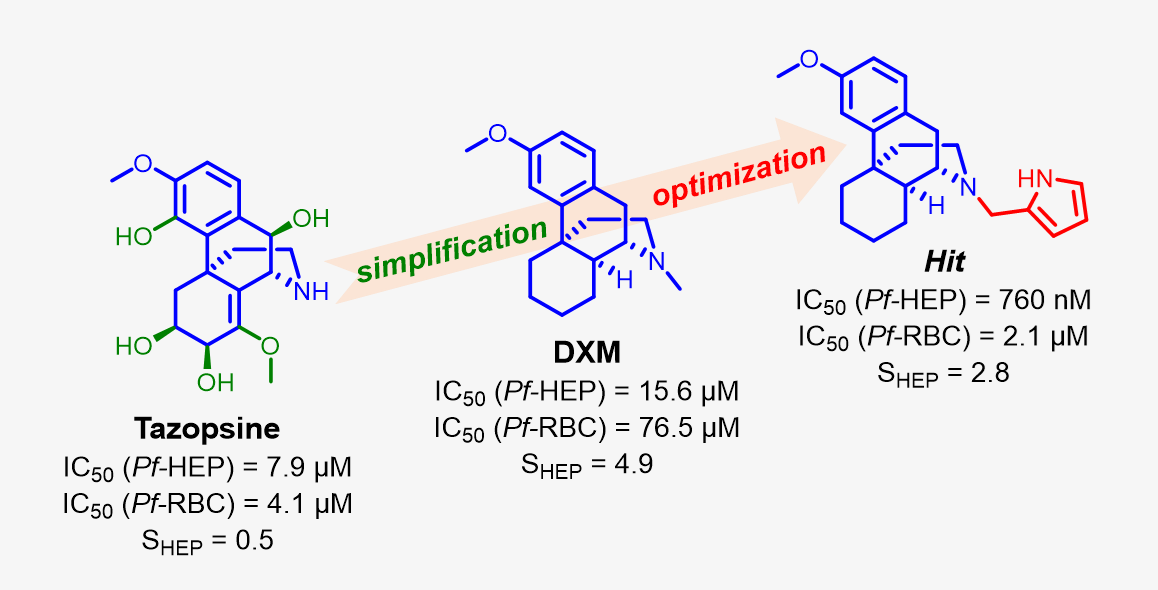
The alkaloid tazopsine 1 was introduced in the late 2000's as a novel antiplasmodial hit compound active against Plasmodium falciparum hepatic stages, with potential to develop prophylactic drugs based on this novel chemical scaffold. However, the structural determinants of tazopsine 1 bioactivity, together with the exact definition of the pharmacophore, remained elusive, impeding further development. We found that the antitussive drug dextromethorphan (DXM) 3, although lacking the complex pattern of stereospecific functionalization of the natural hit, was harboring significant antiplasmodial activity in vitro despite suboptimal prophylactic activity in a murine model of malaria, which precluded its direct repurposing against malaria. The targeted N-alkylation of nor-DXM 15 delivered a small library of analogues with greatly improved activity over DXM 3 against P. falciparum asexual stages. Amongst these, N-2’-pyrrolylmethyl-nor-DXM 16i showed a 2- to 36-fold superior inhibitory potency compared to tazopsine 1 and DXM 3 against parasite liver and blood stages, with 760 ± 130 nM and 2.1 ± 0.4 µM IC50 values, respectively, as well as liver/blood phase selectivity of 2.8. Furthermore, cpd. 16i showed a 5 to 8-fold increase of activity relatively to DXM 3 against P. falciparum stages I-II and V gametocytes, with 18.5 µM and 13.2 µM IC50 values, respectively. Cpd. 16i can thus be considered a promising novel hit compound against malaria in the ent-morphinan series with putative pan-cycle activity, paving the way for further therapeutic development (e. g., investigation of its prophylactic activity in a mouse model of malaria).