Submitted:
09 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
Graphical abstract:
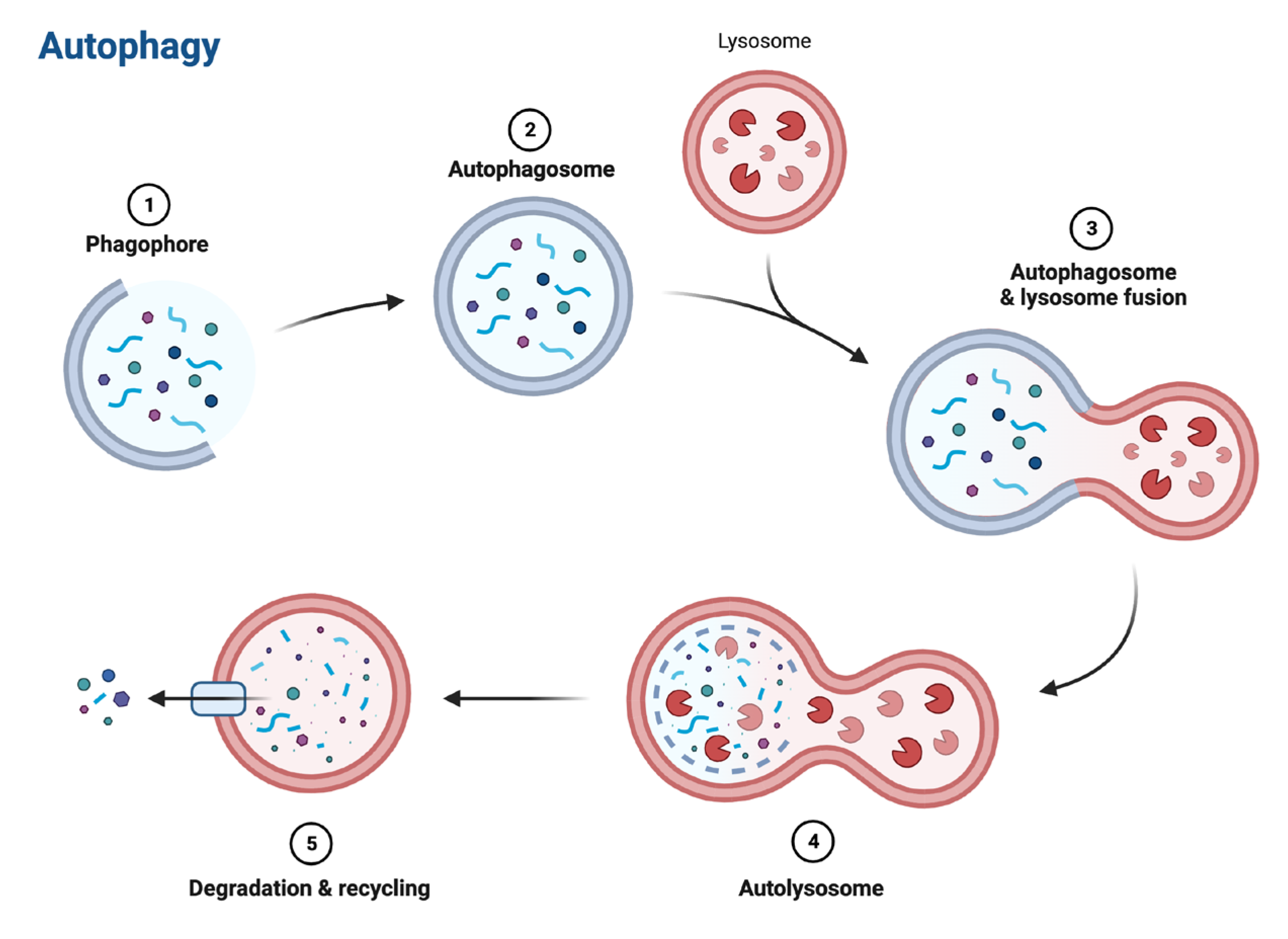
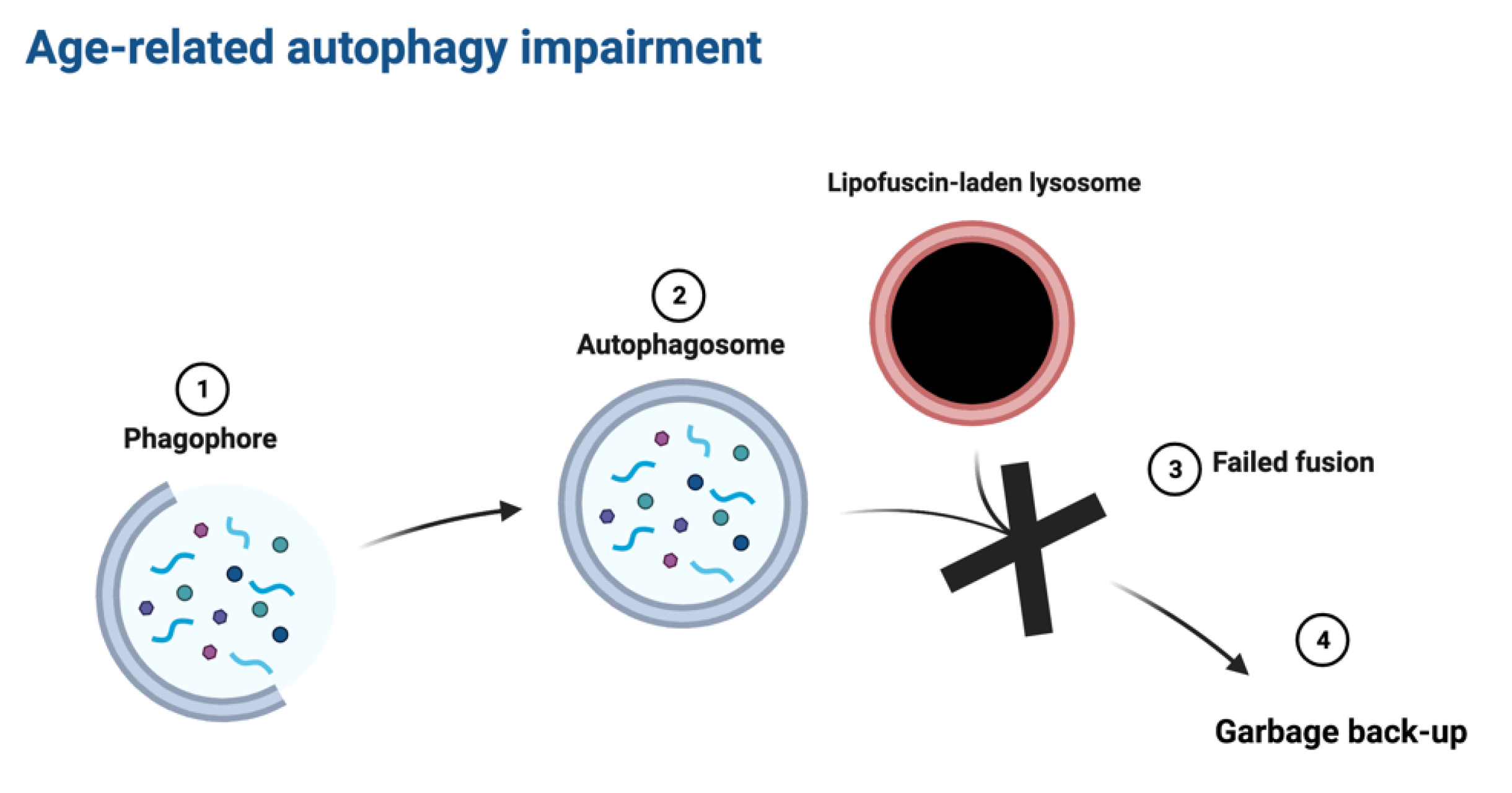
Introduction:
Evolutionary argument:
Mechanistic argument:
Clearance of Undigested Rubbish via Extraction (CURE):
Testing CURE in animal models:
Discussion:
Funding
Authors' contributions
Ethics approval and consent to participate
Consent for publication
Availability of data and material
Acknowledgements
Competing interests
References
- Terman A and Brunk UT. Lipofuscin. The International Journal of Biochemistry & Cell Biology 2004;36(8):1400–1404; [CrossRef]
- Siebert S, Farrell JA, Cazet JF, et al. Stem Cell Differentiation Trajectories in Hydra Resolved at Single-Cell Resolution. Science 2019;365(6451):eaav9314; [CrossRef]
- Murad R, Macias-Muñoz A, Wong A, et al. Coordinated Gene Expression and Chromatin Regulation during Hydra Head Regeneration. Genome Biology and Evolution 2021;13(12):evab221; [CrossRef]
- Terman A, Brunk UT. Is aging the price for memory? Biogerontology (2005) 6:205–210. [CrossRef]
- Aufschnaiter R, Zamir EA, Little CD, Özbek S, Münder S, David CN, et al. In vivo imaging of basement membrane movement: ECM patterning shapes Hydra polyps. Journal of Cell Science 2011;124:4027–38. [CrossRef]
- Klapper W, Kühne K, Singh KK, Heidorn K, Parwaresch R, Krupp G. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Letters (1998) 439:143–146. [CrossRef]
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Structure & Development (2003) 32:39–60. [CrossRef]
- Peregrim I. Why we age — a new evolutionary view. Biologia (2017) 72:475–485. [CrossRef]
- Sheehy M, Shelton P, Wickins J, Belchier M, Gaten E. Ageing the European lobster Homarus gammarus by the lipofuscin in its eyestalk ganglia. Mar Ecol Prog Ser (1996) 143:99–111. [CrossRef]
- Kakimoto Y, Okada C, Kawabe N, Sasaki A, Tsukamoto H, Nagao R, Osawa M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci Rep (2019) 9:3304. [CrossRef]
- Karavanich C, Atema J. Individual recognition and memory in lobster dominance. Animal Behaviour (1998) 56:1553–1560. [CrossRef]
- Zhao S, Lin L, Kan G, Xu C, Tang Q, Yu C, Sun W, Cai L, Xu C, Cui S. High autophagy in the naked mole rat may play a significant role in maintaining good health. Cell Physiol Biochem (2014) 33:321–332. [CrossRef]
- Triplett JC, Tramutola A, Swomley A, Kirk J, Grimes K, Lewis K, Orr M, Rodriguez K, Cai J, Klein JB, et al. Age-related changes in the proteostasis network in the brain of the naked mole-rat: Implications promoting healthy longevity. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease (2015) 1852:2213–2224. [CrossRef]
- Hadi F, Kulaberoglu Y, Lazarus KA, Bach K, Ugur R, Beattie P, Smith ESJ, Khaled WT. Transformation of naked mole-rat cells. Nature (2020) 583:E1–E7. [CrossRef]
- Edrey YH, Hanes M, Pinto M, Mele J, Buffenstein R. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J (2011) 52:41–53. [CrossRef]
- Panno JP, Nair KK. Effects of increased lifespan on chromatin condensation in the adult male housefly. Mech Ageing Dev (1986) 35:31–38. [CrossRef]
- Clokey GV, Jacobson LA. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev (1986) 35:79–94. [CrossRef]
- Houthoofd K, Braeckman BP, Lenaerts I, Brys K, De Vreese A, Van Eygen S, Vanfleteren JR. Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp Gerontol (2002) 37:1015–1021. [CrossRef]
- Goyal VK. Lipofuscin pigment accumulation in the central nervous system of the mouse during aging. Exp Gerontol (1982) 17:89–94. [CrossRef]
- Gilissen EP, Staneva-Dobrovski L. Distinct Types of Lipofuscin Pigment in the Hippocampus and Cerebellum of Aged Cheirogaleid Primates. The Anatomical Record (2013) 296:1895–1906. [CrossRef]
- Moreno-García A, Kun A, Calero O, Medina M, Calero M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Frontiers in Neuroscience (2018) 12: https://www.frontiersin.org/article/10.3389/fnins.2018.00464 [Accessed March 31, 2022].
- Gray DA and Woulfe J. Lipofuscin and Aging: A Matter of Toxic Waste. Science of Aging Knowledge Environment 2005;2005(5):re1–re1; [CrossRef]
- Terman A, Brunk UT. Is Lipofuscin Eliminated from Cells? Investigative Ophthalmology & Visual Science (1999) 40:2463–2464.
- Wang L, Xiao C-Y, Li J-H, Tang G-C, Xiao S-S. Transport and Possible Outcome of Lipofuscin in Mouse Myocardium. Adv Gerontol 2022;12:247–63. [CrossRef]
- Burns JC, Cotleur B, Walther DM, Bajrami B, Rubino SJ, Wei R, Franchimont N, Cotman SL, Ransohoff RM, Mingueneau M. Differential accumulation of storage bodies with aging defines discrete subsets of microglia in the healthy brain. eLife (2020) 9:e57495. [CrossRef]
- Boellaard JW and Schlote W. Ultrastructural Heterogeneity of Neuronal Lipofuscin in the Normal Human Cerebral Cortex. Acta Neuropathol 1986;71(3–4):285–294; [CrossRef]
- Sohal RS, Wolfe LS. Chapter 11 Lipofuscin: characteristics and significance. In: Swaab DF, Fliers E, Mirmiran M, Van Gool WA, Van Haaren F, editors. Progress in Brain Research, vol. 70, Elsevier; 1986, p. 171–83. [CrossRef]
- Sheehy MRJ. Individual variation in, and the effect of rearing temperature and body size on, the concentration of fluorescent morphological lipofuscin in the brains of freshwater crayfish, Cherax cuspidatus (Crustacea: Parastacidae). Comparative Biochemistry and Physiology Part A: Physiology 1990;96:281–6. [CrossRef]
- Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med (2002) 33:611–619. [CrossRef]
- Kurz T, Terman A, Gustafsson B, et al. Lysosomes and oxidative stress in aging and apoptosis. Biochimica et Biophysica Acta (BBA) - General Subjects 2008;1780(11):1291–1303; [CrossRef]
- Gabandé-Rodríguez E, Keane L, Capasso M. Microglial phagocytosis in aging and Alzheimer’s disease. Journal of Neuroscience Research 2020;98(2):284–298; [CrossRef]
- Pan C, Banerjee K, Lehmann GL, et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proceedings of the National Academy of Sciences 2021;118(47):e2100122118; [CrossRef]
- Terman A, Dalen H, Brunk UT. Ceroid/lipofuscin-loaded human fibroblasts show decreased survival time and diminished autophagocytosis during amino acid starvation. Experimental Gerontology 1999;34(8):943–957; [CrossRef]
- Terman A, Abrahamsson N, Brunk UT. Ceroid/lipofuscin-loaded human fibroblasts show increased susceptibility to oxidative stress. Exp Gerontol 1999;34(6):755–770; [CrossRef]
- Lv Z, Jiang H, Xu H, et al. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson’s disease. J Neural Transm 2011;118(3):361–369; [CrossRef]
- Maccarinelli F, Pagani A, Cozzi A, et al. A novel neuroferritinopathy mouse model (FTL 498InsTC) shows progressive brain iron dysregulation, morphological signs of early neurodegeneration and motor coordination deficits. Neurobiology of Disease 2015;81:119–133; [CrossRef]
- Bhoiwala D, Song Y, Cwanger A, et al. High iron diet causes elevation of retinal iron levels and RPE autofluorescence. Investigative Ophthalmology & Visual Science 2015;56(7):4203.
- Mangan D. Iron: an underrated factor in aging. Aging 2021;13(19):23407–23415; [CrossRef]
- Ohgami N, Yajima I, Iida M, et al. Manganese-mediated acceleration of age-related hearing loss in mice. Sci Rep 2016;6(1):36306; [CrossRef]
- Höhn A, Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol 2013;1(1):140–144; [CrossRef]
- von Zglinicki T, Nilsson E, Döcke WD, et al. Lipofuscin accumulation and ageing of fibroblasts. Gerontology 1995;41 Suppl 2:95–108; [CrossRef]
- Tsakiri EN, Iliaki KK, Höhn A, et al. Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radic Biol Med 2013;65:1155–1163; [CrossRef]
- Mann DM, Yates PO, Stamp JE. The relationship between lipofuscin pigment and ageing in the human nervous system. J Neurol Sci (1978) 37:83–93. [CrossRef]
- Goyal VK. Lipofuscin pigment accumulation in human brain during aging. Experimental Gerontology 1982;17(6):481–487; [CrossRef]
- Benavides SH, Monserrat AJ, Fariña S, et al. Sequential histochemical studies of neuronal lipofuscin in human cerebral cortex from the first to the ninth decade of life. Archives of Gerontology and Geriatrics 2002;34(3):219–231; [CrossRef]
- Yin D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radical Biology and Medicine 1996;21(6):871–888; [CrossRef]
- Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investigative Ophthalmology & Visual Science (1978) 17:601–607.
- Dayan D, Abrahami I, Buchner A, Gorsky M, Chimovitz N. Lipid pigment (lipofuscin) in human perioral muscles with aging. Experimental Gerontology (1988) 23:97–102. [CrossRef]
- Xu H, Ren D. Lysosomal Physiology. Annu Rev Physiol (2015) 77:57–80. [CrossRef]
- Kang, Y.-K., Min, B., Eom, J. & Park, J. S. Different phases of aging in mouse old skeletal muscle. Aging (Albany NY) 14, 143–160 (2022).
- Vida C, de Toda IM, Cruces J, et al. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol 2017;12:423–437; [CrossRef]
- Dick SA, Wong A, Hamidzada H, Nejat S, Nechanitzky R, Vohra S, et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Science Immunology 2022;7:eabf7777. [CrossRef]
- Gustafsson K, Rhee C, Frodermann V, Scadden EW, Li D, Iwamoto Y, et al. Clearing and replacing tissue-resident myeloid cells with an anti-CD45 antibody–drug conjugate. Blood Advances 2023;7:6964–73. [CrossRef]
- Rahmberg AR, Wu C, Shin T, Hong SG, Pei L, Markowitz TE, et al. Ongoing production of tissue-resident macrophages from hematopoietic stem cells in healthy adult macaques. Blood Adv 2023;8:523–37. [CrossRef]
- Fischer K, Kraner-Scheiber S, Petersen B, Rieblinger B, Buermann A, Flisikowska T, et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Scientific Reports 2016;6:29081. [CrossRef]
- Wang H, Georgakopoulou A, Zhang W, Kim J, Gil S, Ehrhardt A, et al. HDAd6/35++ - A new helper-dependent adenovirus vector platform for in vivo transduction of hematopoietic stem cells. Molecular Therapy Methods & Clinical Development 2023;29:213–26. [CrossRef]
- Sung CYW, Hayase N, Yuen PST, Lee J, Fernandez K, Hu X, et al. Macrophage Depletion Protects Against Cisplatin-Induced Ototoxicity and Nephrotoxicity. bioRxiv 2023:2023.11.16.567274. [CrossRef]
- Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat Commun 2018;9:4845. [CrossRef]
- Hillmer AT, Holden D, Fowles K, Nabulsi N, West BL, Carson RE, et al. Microglial depletion and activation: A [11C]PBR28 PET study in nonhuman primates. EJNMMI Res 2017;7:59. [CrossRef]
- Green KN, Crapser JD, Hohsfield LA. To Kill Microglia: A Case for CSF1R Inhibitors. Trends Immunol 2020;41:771–84. [CrossRef]
- Chadarevian JP, Lombroso SI, Peet GC, Hasselmann J, Tu C, Marzan DE, et al. Engineering an inhibitor-resistant human CSF1R variant for microglia replacement. Journal of Experimental Medicine 2022;220:e20220857. [CrossRef]
- Claeys W, Verhaege D, Van Imschoot G, Van Wonterghem E, Van Acker L, Amelinck L, et al. Limitations of PLX3397 as a microglial investigational tool: peripheral and off-target effects dictate the response to inflammation. Front Immunol 2023;14:1283711. [CrossRef]
- Shaikh SN, Willis EF, Dierich M, Xu Y, Stuart SJS, Gobe GC, et al. CSF-1R inhibitor PLX3397 attenuates peripheral and brain chronic GVHD and improves functional outcomes in mice. J Neuroinflammation 2023;20:300. [CrossRef]
- Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol 2016;18:557–64. [CrossRef]
- MacDonald KPA, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 2010;116:3955–63. [CrossRef]
- Hayal TB, Wu C, Abraham D, Demirci S, Palchaudhuri R, Lanieri L, et al. The Impact of CD45-Antibody-Drug Conjugate Conditioning on Clonal Dynamics and Immune Tolerance Post HSPC Transplantation in Rhesus Macaques. Blood 2023;142:3419. [CrossRef]
- Wellhausen N, O’Connell RP, Lesch S, Engel NW, Rennels AK, Gonzales D, et al. Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy. Sci Transl Med 2023;15:eadi1145. [CrossRef]
- Picco G, Petti C, Trusolino L, Bertotti A, Medico E. A diphtheria toxin resistance marker for in vitro and in vivo selection of stably transduced human cells. Sci Rep 2015;5:14721. [CrossRef]
- Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. The EMBO Journal 2017;36:42–60. [CrossRef]
- Insall RH, Paschke P, Tweedy L. Steering yourself by the bootstraps: how cells create their own gradients for chemotaxis. Trends in Cell Biology 2022;32:585–96. [CrossRef]
- Tigges M, Marquez-Lago TT, Stelling J, Fussenegger M. A tunable synthetic mammalian oscillator. Nature 2009;457:309–12. [CrossRef]
- Choi I, Kim B, Byun J-W, Baik SH, Huh YH, Kim J-H, et al. LRRK2 G2019S mutation attenuates microglial motility by inhibiting focal adhesion kinase. Nat Commun 2015;6:8255. [CrossRef]
- Russo I, Bubacco L, Greggio E. LRRK2 as a target for modulating immune system responses. Neurobiology of Disease 2022;169:105724. [CrossRef]
- Kitchen GB, Cunningham PS, Poolman TM, Iqbal M, Maidstone R, Baxter M, et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc Natl Acad Sci U S A 2020;117:1543–51. [CrossRef]
- Horns F, Martinez JA, Fan C, Haque M, Linton JM, Tobin V, et al. Engineering RNA export for measurement and manipulation of living cells. Cell 2023;186:3642-3658.e32. [CrossRef]
- Birgisdottir ÅB, Lamark T, Johansen T. The LIR motif - crucial for selective autophagy. J Cell Sci 2013;126(Pt 15):3237–3247; [CrossRef]
- Martinelli S, Anderzhanova EA, Bajaj T, Wiechmann S, Dethloff F, Weckmann K, et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat Commun 2021;12:4643. [CrossRef]
- Uematsu M, Nishimura T, Sakamaki Y, et al. Accumulation of undegraded autophagosomes by expression of dominant-negative STX17 (syntaxin 17) mutants. Autophagy 2017;13(8):1452–1464; [CrossRef]
- Tan HWS, Lu G, Dong H, Cho Y-L, Natalia A, Wang L, et al. A degradative to secretory autophagy switch mediates mitochondria clearance in the absence of the mATG8-conjugation machinery. Nat Commun 2022;13:3720. [CrossRef]
- Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, et al. Chimeric antigen receptors that trigger phagocytosis. eLife 2018;7:e36688. [CrossRef]
- Friedland AE, Lu TK, Wang X, Shi D, Church G, Collins JJ. Synthetic Gene Networks that Count. Science 2009;324:1199–202. [CrossRef]
- VanHook AM. Macrophages don’t take more than they can eat. Science Signaling 2017;10:eaao1183. [CrossRef]
- Watson JL, Krüger LK, Ben-Sasson AJ, Bittleston A, Shahbazi MN, Planelles-Herrero VJ, et al. Synthetic Par polarity induces cytoskeleton asymmetry in unpolarized mammalian cells. Cell 2023;186:4710-4727.e35. [CrossRef]
- Loeffler D, Wehling A, Schneiter F, Zhang Y, Müller-Bötticher N, Hoppe PS, et al. Asymmetric lysosome inheritance predicts activation of haematopoietic stem cells. Nature 2019;573:426–9. [CrossRef]
- Ronda C, Chen SP, Cabral V, Yaung SJ, Wang HH. Metagenomic engineering of the mammalian gut microbiome in situ. Nat Methods 2019;16:167–70. [CrossRef]
- Wang X, Maxwell KG, Wang K, Bowers DT, Flanders JA, Liu W, et al. A nanofibrous encapsulation device for safe delivery of insulin-producing cells to treat type 1 diabetes. Sci Transl Med 2021;13:eabb4601. [CrossRef]
- Park JS, Rhau B, Hermann A, McNally KA, Zhou C, Gong D, et al. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc Natl Acad Sci USA 2014;111:5896–901. [CrossRef]
- Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci 2020;23:1157–67. [CrossRef]
- Italiani P, Boraschi D. New Insights Into Tissue Macrophages: From Their Origin to the Development of Memory. Immune Netw 2015;15:167–76. [CrossRef]
- Wang SK, Cepko CL. Targeting Microglia to Treat Degenerative Eye Diseases. Front Immunol 2022;13. [CrossRef]
- Streeter MD, Rowan S, Ray J, et al. Generation and Characterization of Anti-Glucosepane Antibodies Enabling Direct Detection of Glucosepane in Retinal Tissue. ACS Chem Biol 2020;15(10):2655–2661; [CrossRef]
- Scheller L, Strittmatter T, Fuchs D, Bojar D, Fussenegger M. Generalized extracellular molecule sensor platform for programming cellular behavior. Nat Chem Biol 2018;14:723–9. [CrossRef]
- Seluanov A, Gladyshev VN, Vijg J, et al. Mechanisms of cancer resistance in long-lived mammals. Nat Rev Cancer 2018;18(7):433–441; [CrossRef]
- Samorajski T, Ordy JM, Rady-Reimer P. Lipofuscin pigment accumulation in the nervous system of aging mice. The Anatomical Record (1968) 160:555–573. [CrossRef]
- Brizzee KR, Johnson FA. Depth distribution of lipofuscin pigment in cerebral cortex of albino rat. Acta Neuropathol (1970) 16:205–219. [CrossRef]
- Yanai S, Endo S. Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype. Frontiers in Aging Neuroscience 2021;13.
- Lutz CM, Osborne MA. Optimizing mouse models of neurodegenerative disorders: are therapeutics in sight? Future Neurology 2014;9(1):67–75; [CrossRef]
- Double KL, Dedov VN, Fedorow H, et al. The comparative biology of neuromelanin and lipofuscin in the human brain. Cell Mol Life Sci 2008;65(11):1669–1682; [CrossRef]
- Spampanato C, Feeney E, Li L, Cardone M, Lim J-A, Annunziata F, et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med 2013;5:691–706. [CrossRef]
- Abascal F, Harvey LMR, Mitchell E, Lawson ARJ, Lensing SV, Ellis P, et al. Somatic mutation landscapes at single-molecule resolution. Nature 2021;593:405–10. [CrossRef]
- Cortese FAB, Santostasi G. Whole-Body Induced Cell Turnover: A Proposed Intervention for Age-Related Damage and Associated Pathology. Rejuvenation Res 2016;19:322–36. [CrossRef]
- Zealley B, de Grey ADNJ. Strategies for Engineered Negligible Senescence. Gerontology 2013;59:183–9. [CrossRef]
- Yoon YG, Koob MD. Transformation of isolated mammalian mitochondria by bacterial conjugation. Nucleic Acids Res 2005;33:e139. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



