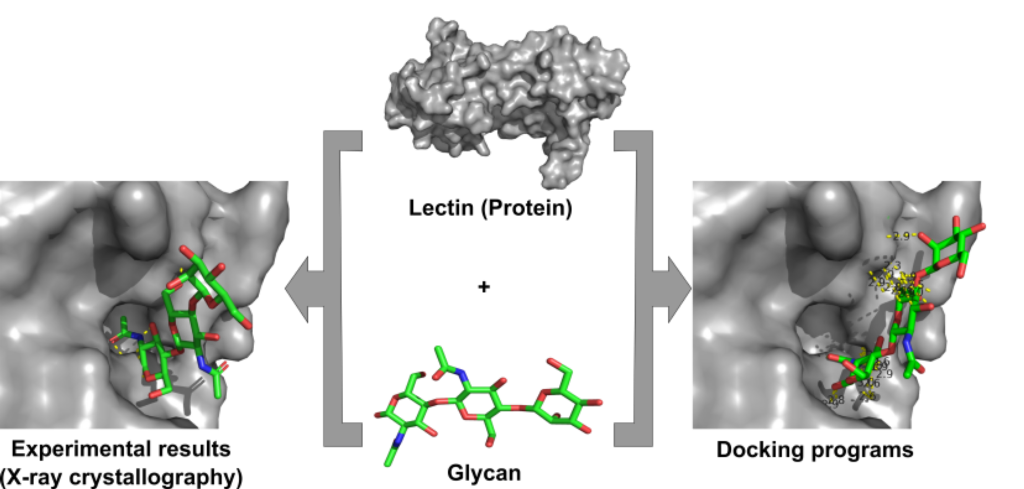
The Severe acute respiratory syndrome-Corona virus-2 (SARS-CoV-2) which is responsible for recurring pandemics takes advantage of host-cell processes including the glycosylation pathway. The heavily glycosylated spike protein assists viruses in attachment and penetration. The N-glycans of N-terminal domain N165 and N234 play a significant role in conformational dynamics of the receptor-binding domain (RBD) with angiotensin-converting enzyme 2 (ACE2). In addition, the deletion of N-glycan sites N331 and N334 have been associated with reduced infectivity. This signifies the importance of targeting the N-glycans making them unavailable for interacting with the ACE2 receptor, ultimately leading to reduced infectivity. These glycans can be specifically targeted and can be used for designing SARS specific drugs or neutralizing molecules. In the current study, lectins Griffithsin, Cyanovirin-N, Cyanovirin homolog, and BanLec H84T were used to target the spike protein's N-glycans. Molecular docking programs AutoDock Vina and HADDOCK were used to study lectin-glycan interactions. The interactions look convincible in accordance with the lowest interaction energies best-fit approach but the characteristic feature (scoring functions) of the docking programs are questionable concerning Lectin-Glycan interactions exist in nature. There is a high need of the hour for developing specific algorithms for docking glycans with a preferential selection of terminal residues.