Submitted:
10 January 2023
Posted:
11 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
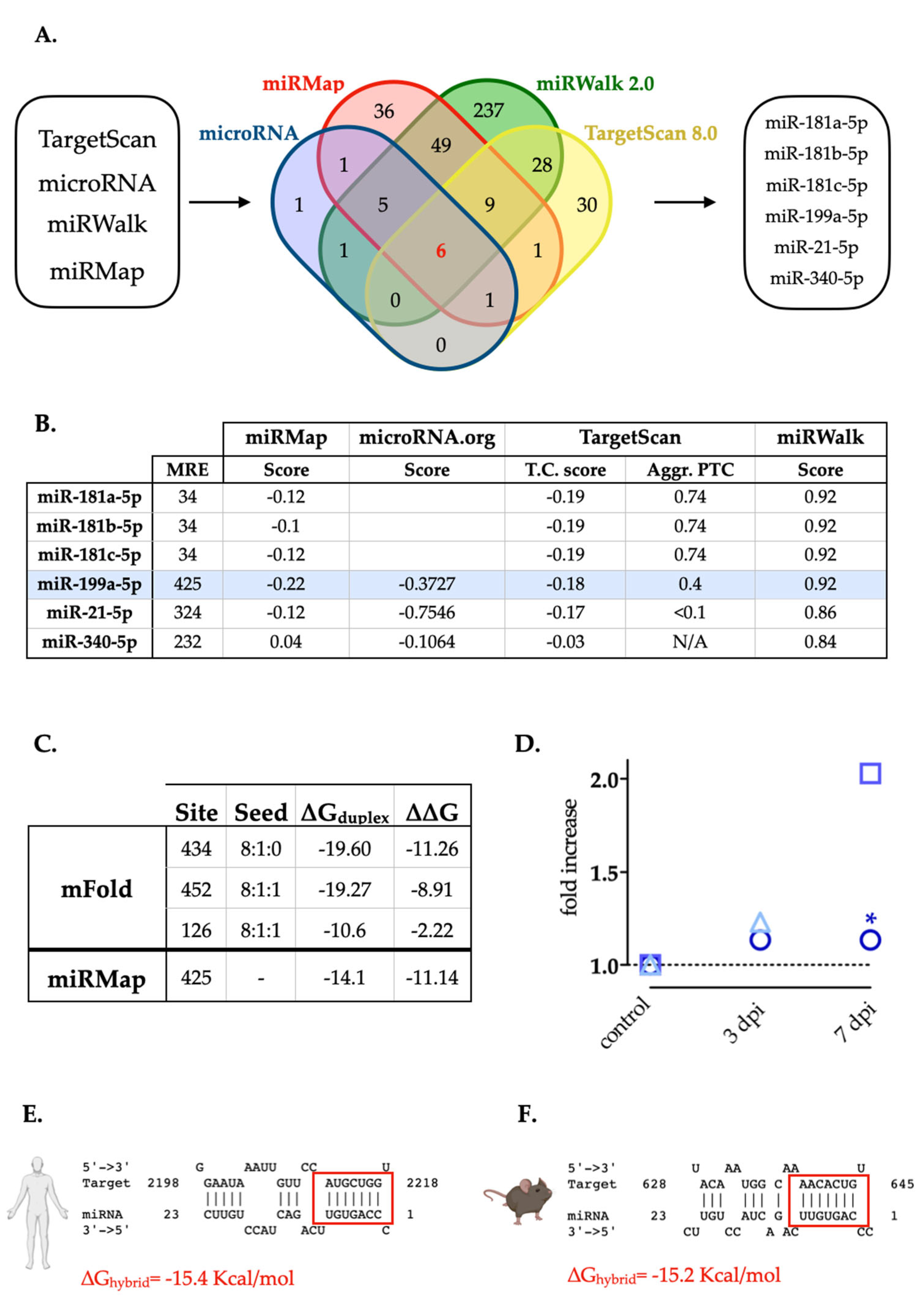
2. Results
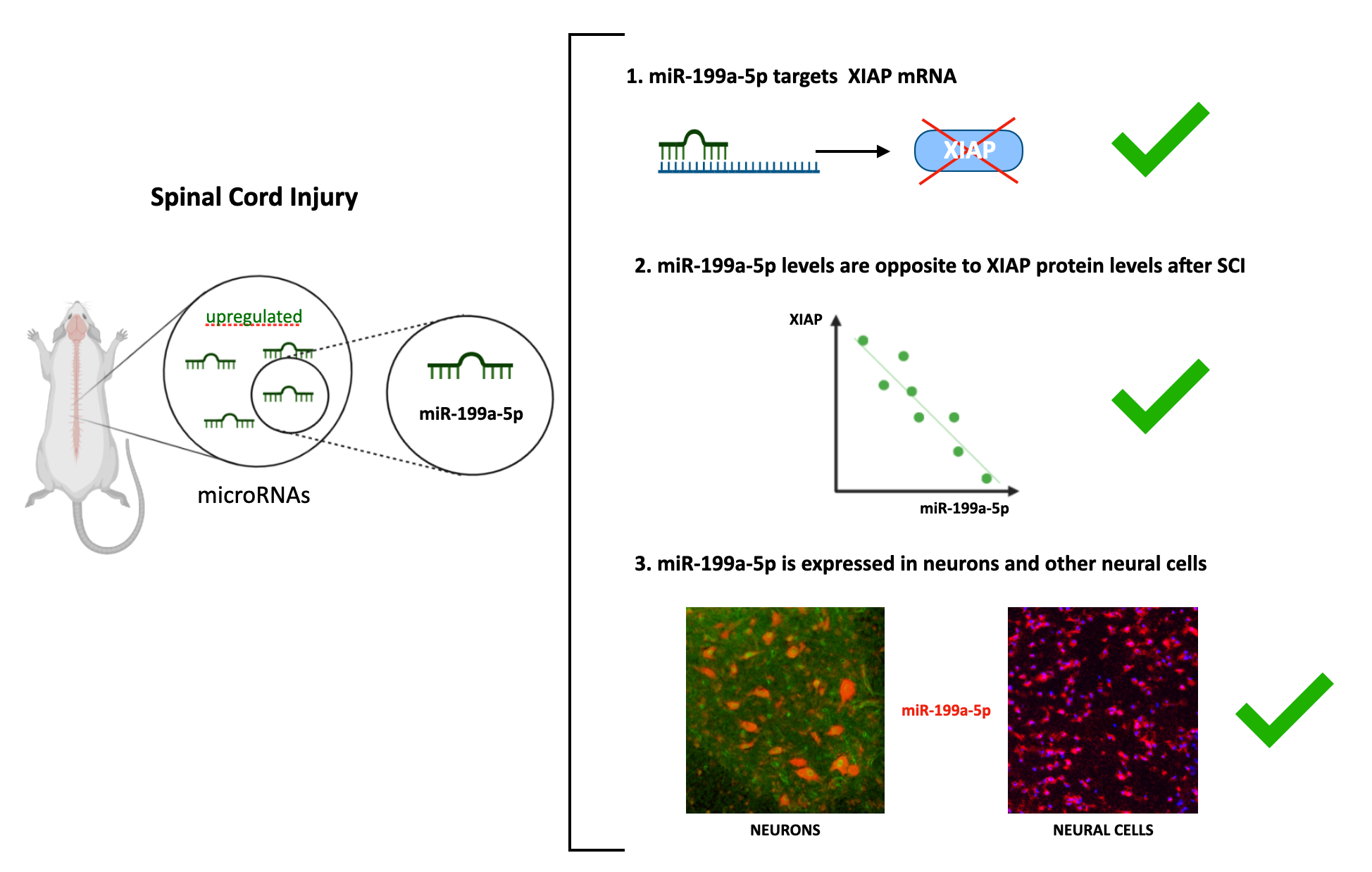
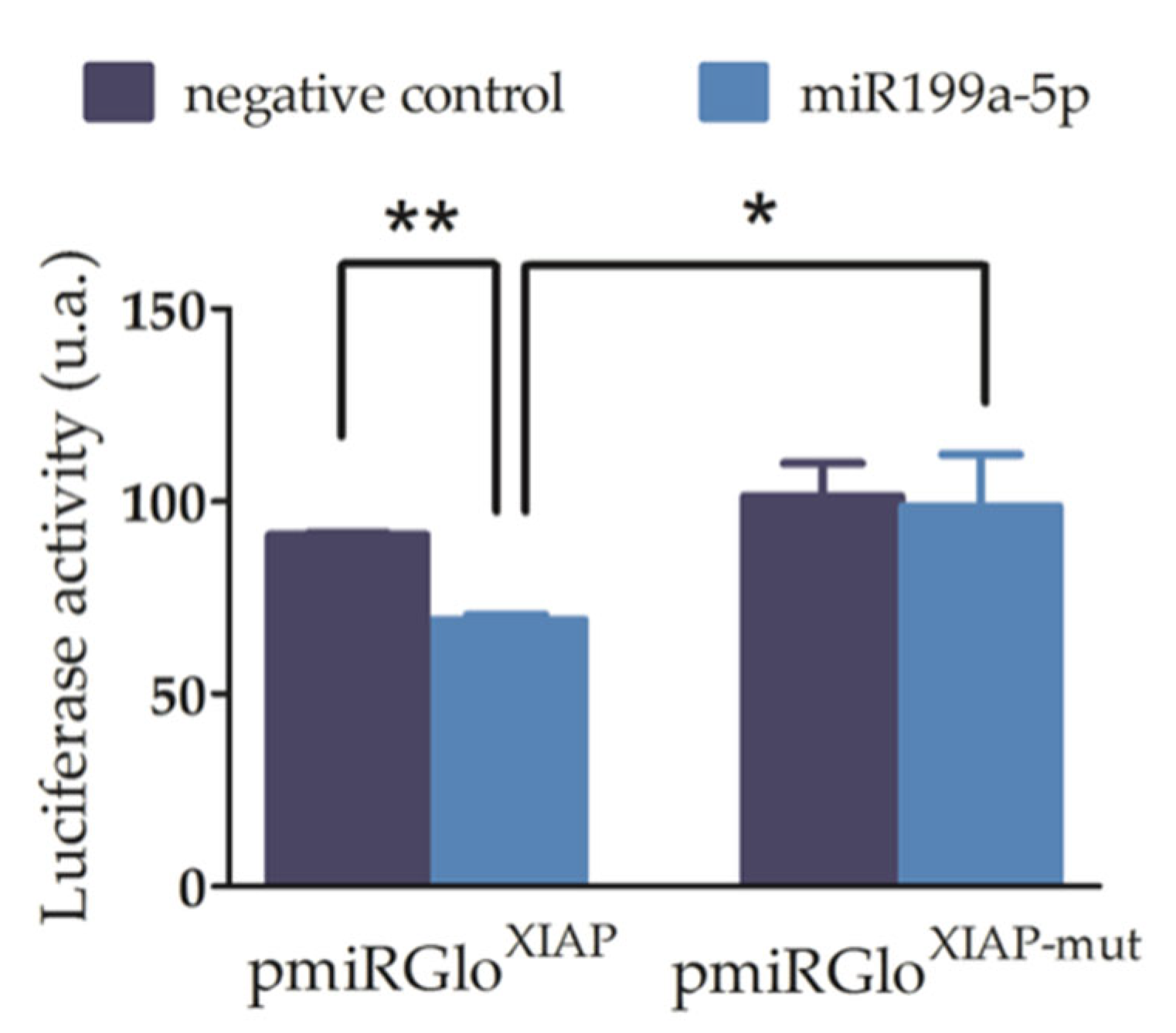
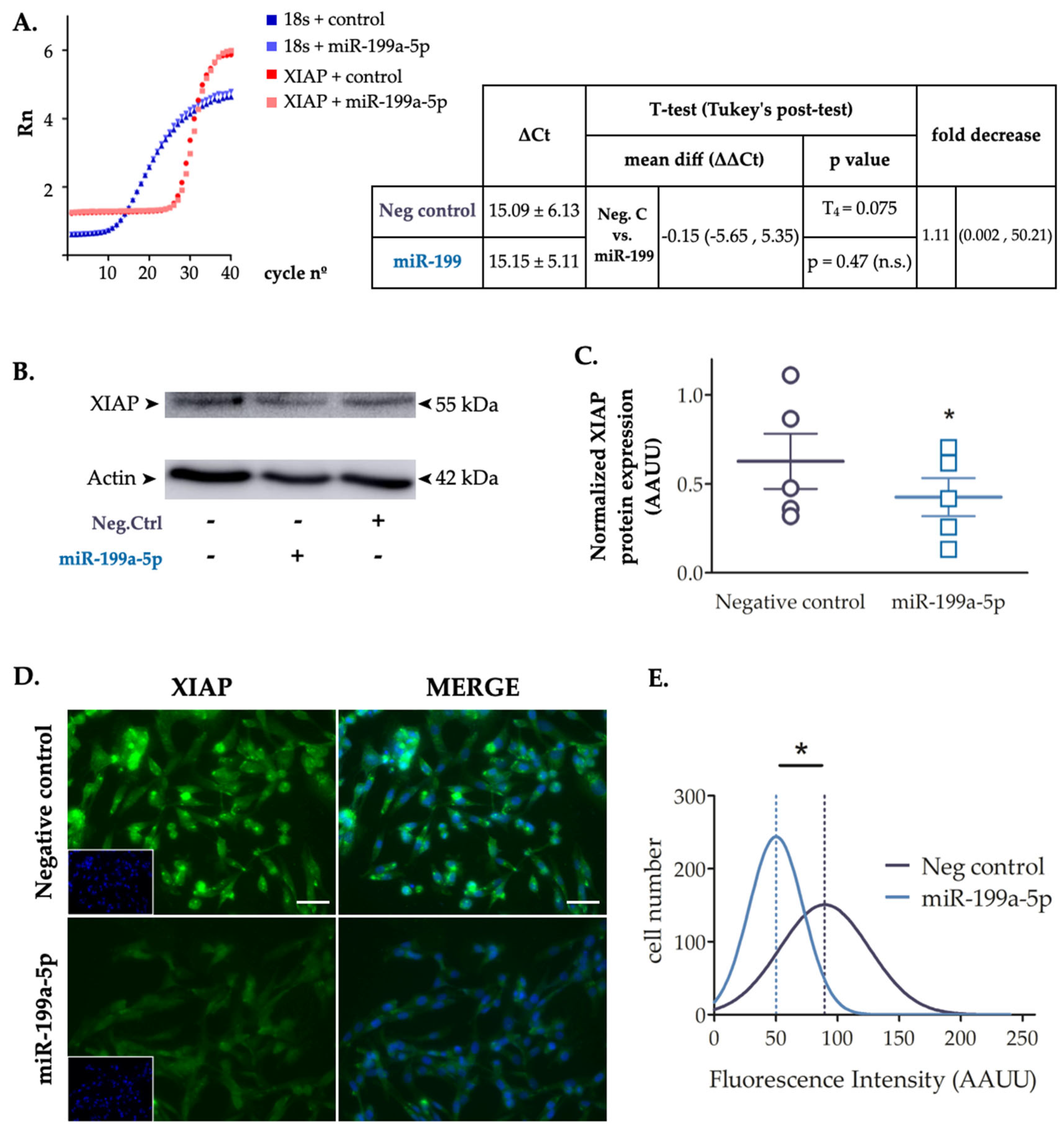
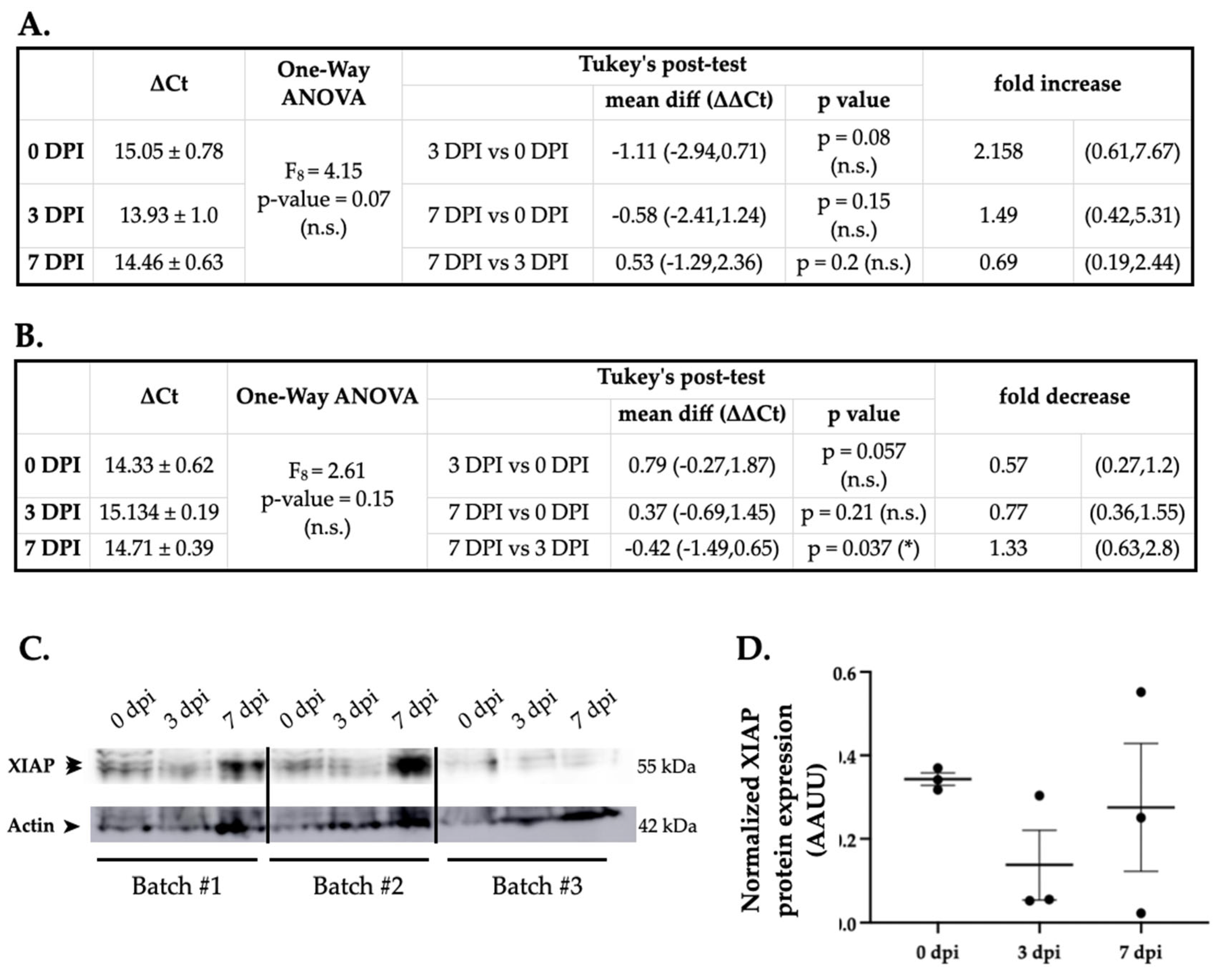
2.3. Increased Levels of miR-199a-5p Reduce XIAP Protein Expression
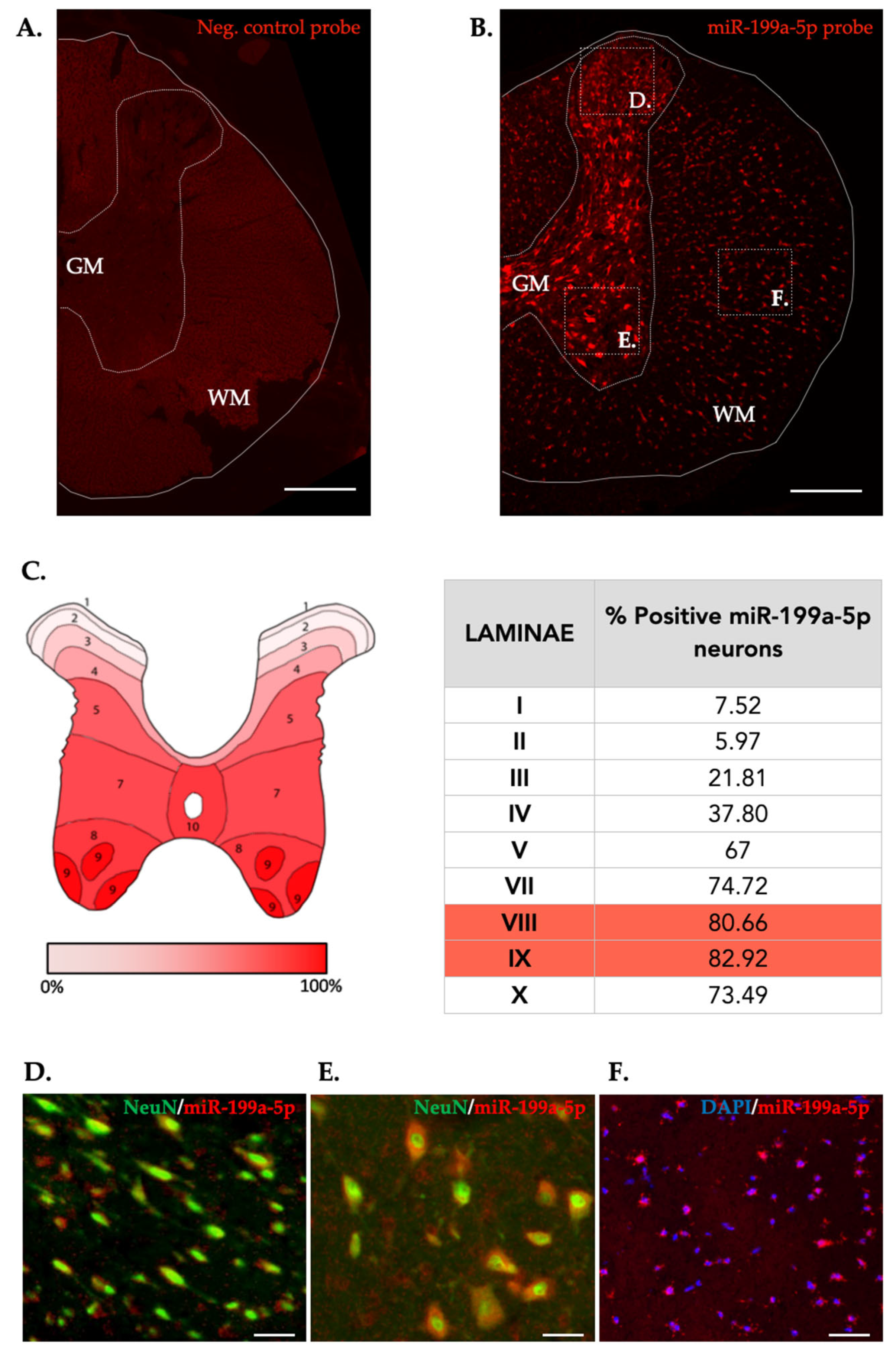
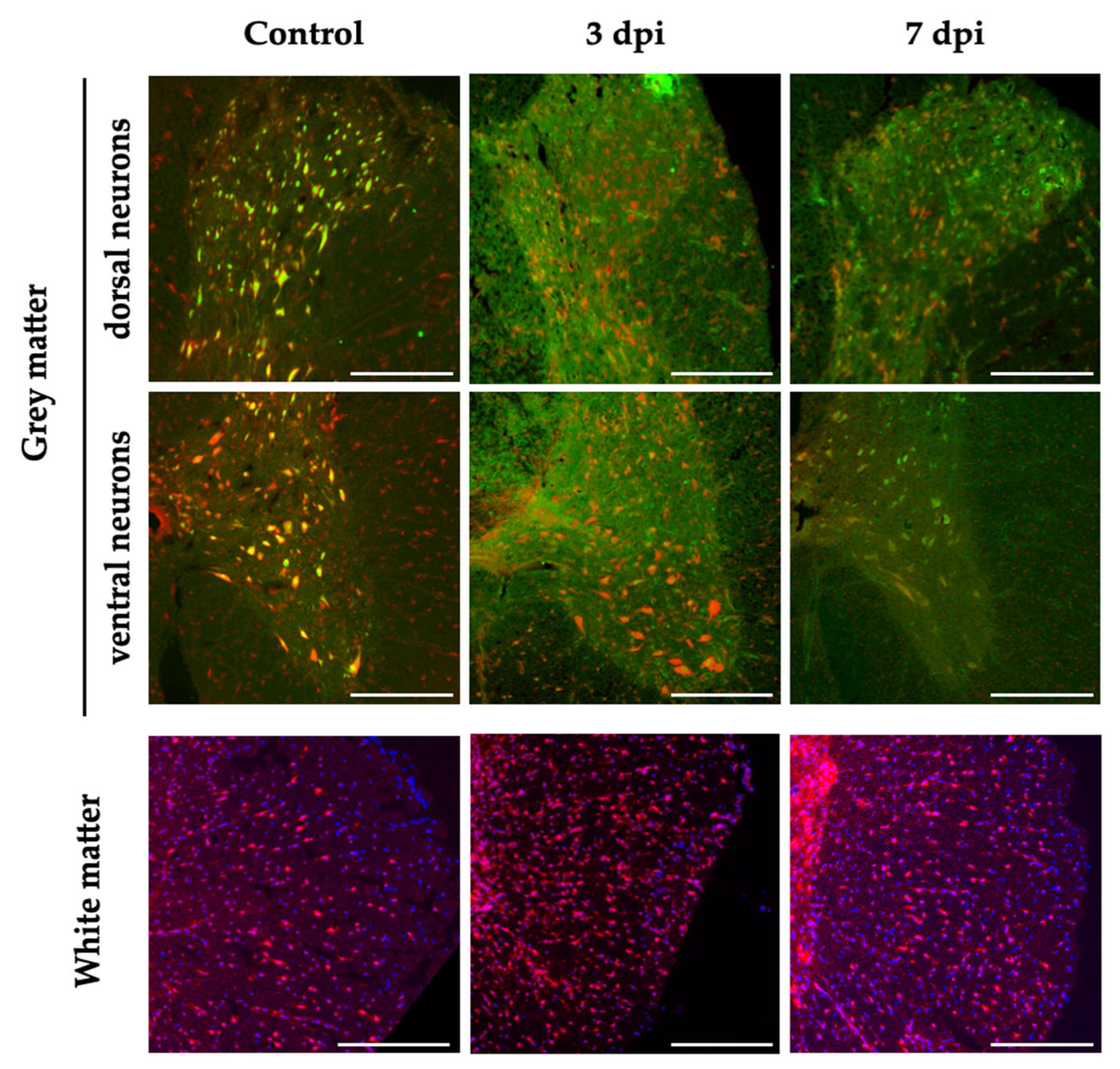
2.4. Changes in miR-199a-5p Expression after SCI and Their Relation with XIAP Expression
3. Discussion
4. Materials and Methods
4.1. Bioinformatics and Data Mining
4.2. Spinal Cord Injury Model
4.3. Cell Culture
4.4. Transfections and Sample Collection
4.5. RT-PCR
4.6. Dual-Luciferase Reporter Gene Construction and 3’UTR Luciferase Reporter Assays
4.7. Immunoblotting Assay
4.8. Immunofluorescence
4.9. Fluorescence in situ Hybridization (FISH)
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devivo, M. J. 2012. Epidemiology of traumatic spinal cord injury: Trends and future implications. In Spinal Cord, 50:365–372. Spinal Cord. [CrossRef]
- Alizadeh, Arsalan, Scott Matthew Dyck, and Soheila Karimi-Abdolrezaee. 2019. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Frontiers in Neurology. [CrossRef]
- Ahuja, Christopher S., Jefferson R. Wilson, Satoshi Nori, Mark R. N. Kotter, Claudia Druschel, Armin Curt, and Michael G. Fehlings. 2017. Traumatic spinal cord injury. Nature Reviews Disease Primers 3. Nature Publishing Group: 1–21. [CrossRef]
- Grossman, S. D., L. J. Rosenberg, and J. R. Wrathall. 2001. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Experimental Neurology 168: 273–282. [CrossRef]
- Barbon, Alessandro, Fabio Fumagalli, Luca Caracciolo, Laura Madaschi, Elena Lesma, Cristina Mora, Stephana Carelli, et al. 2010. Acute spinal cord injury persistently reduces R/G RNA editing of AMPA receptors. Journal of Neurochemistry 114: 397–407. [CrossRef]
- Nakae, Aya, Kunihiro Nakai, Tatsuya Tanaka, Ko Hosokawa, and Takashi Mashimo. 2013. Serotonin 2C receptor alternative splicing in a spinal cord injury model. Neuroscience Letters 532: 49–54. [CrossRef]
- 7. Anilkumar, Ujval, and Jochen H. M. Prehn. 2014. Anti-apoptotic BCL-2 family proteins in acute neural injury. Frontiers in Cellular Neuroscience 8. Frontiers Research Foundation: 281. [CrossRef]
- Dasari, Venkata Ramesh, Krishna Kumar Veeravalli, Andrew J. Tsung, Christopher S. Gondi, Meena Gujrati, Dzung H. Dinh, and Jasti S. Rao. 2009. Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. Journal of Neurotrauma 26. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA: 2057–2069. [CrossRef]
- Tu, Huailu, and Max Costa. 2020. XIAP’s Profile in Human Cancer. Biomolecules 10: 1493. [CrossRef]
- Roy, N., Q. L. Deveraux, R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. The EMBO journal 16: 6914–6925. [CrossRef]
- Hollville, Emilie, Selena E. Romero, and Mohanish Deshmukh. 2019. Apoptotic cell death regulation in neurons. The FEBS Journal 286. Blackwell Publishing Ltd: 3276–3298. [CrossRef]
- Zhang, Ning, Ying Yin, Sheng-Jie Xu, Yong-Ping Wu, and Wei-Shan Chen. 2012. Inflammation & apoptosis in spinal cord injury. The Indian Journal of Medical Research 135: 287–296.
- Kang, Young Ji, Mi Jang, Yun Kyung Park, Sunghyun Kang, Kwang-Hee Bae, Sayeon Cho, Chong-Kil Lee, Byoung Chul Park, Seung-Wook Chi, and Sung Goo Park. 2010. Molecular interaction between HAX-1 and XIAP inhibits apoptosis. Biochemical and Biophysical Research Communications 393: 794–799. [CrossRef]
- Xu, Jiheng, Xiaohui Hua, Rui Yang, Honglei Jin, Jingxia Li, Junlan Zhu, Zhongxian Tian, et al. 2019. XIAP Interaction with E2F1 and Sp1 via its BIR2 and BIR3 domains specific activated MMP2 to promote bladder cancer invasion. Oncogenesis 8. Nature Publishing Group: 1–9. [CrossRef]
- Harlin, H., S. B. Reffey, C. S. Duckett, T. Lindsten, and C. B. Thompson. 2001. Characterization of XIAP-deficient mice. Molecular and Cellular Biology 21: 3604–3608. [CrossRef]
- 16. Blancas, Sugela, Rut Fadó, José Rodriguez-Alvarez, and Julio Morán. 2014. Endogenous XIAP, but not other members of the inhibitory apoptosis protein family modulates cerebellar granule neurons survival. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience 37: 26–35. [CrossRef]
- 17. West, Tim, Madeliene Stump, Gregory Lodygensky, Jeff J. Neil, Mohanish Deshmukh, and David M. Holtzman. 2009. Lack of X-linked inhibitor of apoptosis protein leads to increased apoptosis and tissue loss following neonatal brain injury. ASN neuro 1. [CrossRef]
- Potts, Patrick Ryan, Shweta Singh, Malia Knezek, Craig B. Thompson, and Mohanish Deshmukh. 2003. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. Journal of Cell Biology 163. J Cell Biol: 789–799. [CrossRef]
- Perrelet, D., A. Ferri, P. Liston, P. Muzzin, R. G. Korneluk, and A. C. Kato. 2002. IAPs are essential for GDNF-mediated neuroprotective effects in injured motor neurons in vivo. Nature Cell Biology 4. Nature Publishing Group: 175–179. [CrossRef]
- 20. Keane, Robert W., Susan Kraydieh, George Lotocki, John R. Bethea, Stanislaw Krajewski, John C. Reed, and W. Dalton Dietrich. 2001. Apoptotic and Anti-Apoptotic Mechanisms Following Spinal Cord Injury. Journal of Neuropathology & Experimental Neurology 60. American Association of Neuropathologists Inc.: 422–429. [CrossRef]
- Xu, D., Y. Bureau, D. C. McIntyre, D. W. Nicholson, P. Liston, Y. Zhu, W. G. Fong, S. J. Crocker, R. G. Korneluk, and G. S. Robertson. 1999. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 19: 5026–5033.
- de Rivero Vaccari, Juan Pablo, W Dalton Dietrich, and Robert W Keane. 2014. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. Journal of Cerebral Blood Flow & Metabolism 34: 369–375. [CrossRef]
- Reigada, D., M. Nieto-Díaz, R. Navarro-Ruiz, M. J. Caballero-López, A. del Águila, T. Muñoz-Galdeano, and R. M. Maza. 2015. Acute administration of ucf-101 ameliorates the locomotor impairments induced by a traumatic spinal cord injury. Neuroscience 300. Elsevier Ltd: 404–417. [CrossRef]
- Overexpression of the X-linked Inhibitor of Apoptosis Protein (XIAP) in Neurons Improves Cell Survival and the Functional Outcome after Traumatic Spinal Cord Injury. 2022. https://scholar.google.es/citations?view_op=view_citation&hl=es&user=AGJqIloAAAAJ&citation_for_view=AGJqIloAAAAJ:hMod-77fHWUC. Accessed December 26.
- MicroRNA Dysregulation in the Spinal Cord following Traumatic Injury. 2021. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0034534. Accessed June 8.
- Nieto-Díaz, Manuel, Francisco José Esteban, David Reigada, Teresa Muñoz-Galdeano, Mónica Yunta, Marcos Caballero-López, Rosa Navarro-Ruiz, Ángela del Águila, and Rodrigo Martínez Maza. 2014. MicroRNA dysregulation in spinal cord injury: causes, consequences and therapeutics. Frontiers in Cellular Neuroscience 8. Frontiers. [CrossRef]
- Selbach, Matthias, Björn Schwanhäusser, Nadine Thierfelder, Zhuo Fang, Raya Khanin, and Nikolaus Rajewsky. 2008. Widespread changes in protein synthesis induced by microRNAs. Nature 455. Nature Publishing Group: 58–63. [CrossRef]
- Sayed, Danish, and Maha Abdellatif. 2011. MicroRNAs in development and disease. Physiological Reviews 91: 827–887. [CrossRef]
- Su, Zhenyi, Zuozhang Yang, Yongqing Xu, Yongbin Chen, and Qiang Yu. 2015. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget 6: 8474–8490. [CrossRef]
- Hata, Akiko, and Judy Lieberman. 2015. Dysregulation of microRNA biogenesis and gene silencing in cancer. Science Signaling 8: re3. [CrossRef]
- Zhang, Haocong, and Yan Wang. 2016. Identification of molecular pathway changes after spinal cord injury by microarray analysis. Journal of Orthopaedic Surgery and Research 11. [CrossRef]
- Liu, Yugang, Ying Wang, Zhaowei Teng, Xiufeng Zhang, Min Ding, Zhaojun Zhang, Junli Chen, and Yanli Xu. 2016. DNA Microarray Analysis in Screening Features of Genes Involved in Spinal Cord Injury. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research 22: 1571–1581. [CrossRef]
- Squair, Jordan W, Seth Tigchelaar, Kyung-Mee Moon, Jie Liu, Wolfram Tetzlaff, Brian K Kwon, Andrei V Krassioukov, Christopher R West, Leonard J Foster, and Michael A Skinnider. 2018. Integrated systems analysis reveals conserved gene networks underlying response to spinal cord injury. eLife 7. eLife Sciences Publications, Ltd: e39188. [CrossRef]
- Maza, Rodrigo M., María Asunción Barreda-Manso, David Reigada, Ágata Silván, Teresa Muñoz-Galdeano, Altea Soto, Ángela del Águila, and Manuel Nieto-Díaz. 2022. MicroRNA-138-5p Targets Pro-Apoptotic Factors and Favors Neural Cell Survival: Analysis in the Injured Spinal Cord. Biomedicines 10. Multidisciplinary Digital Publishing Institute: 1559. [CrossRef]
- Xu, Zhongyang, Kefeng Zhang, Qian Wang, and Yanping Zheng. 2019. MicroRNA-124 improves functional recovery and suppresses Bax-dependent apoptosis in rats following spinal cord injury. Molecular Medicine Reports 19: 2551–2560. [CrossRef]
- Jiang, Dongdong, Fangyi Gong, Xuhui Ge, Chengtang Lv, Chenyu Huang, Shuang Feng, Zheng Zhou, et al. 2020. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. Journal of Nanobiotechnology 18: 105. [CrossRef]
- Sabirzhanov, Boris, Jessica Matyas, Marina Coll-Miro, Laina Lijia Yu, Alan I. Faden, Bogdan A. Stoica, and Junfang Wu. 2019. Inhibition of microRNA-711 limits angiopoietin-1 and Akt changes, tissue damage, and motor dysfunction after contusive spinal cord injury in mice. Cell Death & Disease 10. Nature Publishing Group: 1–14. [CrossRef]
- Sabirzhanov, Boris, Zaorui Zhao, Bogdan A. Stoica, David J. Loane, Junfang Wu, Carlos Borroto, Susan G. Dorsey, and Alan I. Faden. 2014. Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 34: 10055–10071. [CrossRef]
- Liu, Xing, Xintao Cui, Guangwei Guan, Ying Dong, and Zhenyu Zhang. 2020. microRNA-192-5p is involved in nerve repair in rats with peripheral nerve injury by regulating XIAP. Cell Cycle 19. Taylor and Francis Inc.: 326–338. [CrossRef]
- Siegel, Chad, Jun Li, Fudong Liu, Sharon E. Benashski, and Louise D. McCullough. 2011. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America 108. National Academy of Sciences: 11662–11667. [CrossRef]
- Rennie, William, Chaochun Liu, C. Steven Carmack, Adam Wolenc, Shaveta Kanoria, Jun Lu, Dang Long, and Ye Ding. 2014. STarMir: a web server for prediction of microRNA binding sites. Nucleic Acids Research 42: W114-118. [CrossRef]
- REHMSMEIER, MARC, PETER STEFFEN, MATTHIAS HÖCHSMANN, and ROBERT GIEGERICH. 2004. Fast and effective prediction of microRNA/target duplexes. RNA 10: 1507–1517. [CrossRef]
- 43. Hutchison, Emmette R., Elisa M. Kawamoto, Dennis D. Taub, Ashish Lal, Kotb Abdelmohsen, Yongqing Zhang, William H. Wood, et al. 2013. Involvement of miR-181 in Neuroinflammatory Responses of Astrocytes. Glia 61: 1018–1028. [CrossRef]
- Chen, Jia-Nan, Yi-Ning Zhang, Li-Ge Tian, Ying Zhang, Xin-Yu Li, and Bin Ning. 2022. Down-regulating Circular RNA Prkcsh suppresses the inflammatory response after spinal cord injury. Neural Regeneration Research 17: 144–151. [CrossRef]
- Liu, Nai-Kui, Xiao-Fei Wang, Qing-Bo Lu, and Xiao-Ming Xu. 2009. Altered microRNA expression following traumatic spinal cord injury. Experimental Neurology 219: 424–429. [CrossRef]
- Yunta, Mónica, Manuel Nieto-Díaz, Francisco J. Esteban, Marcos Caballero-López, Rosa Navarro-Ruíz, David Reigada, D. Wolfgang Pita-Thomas, Ángela del Águila, Teresa Muñoz-Galdeano, and Rodrigo M. Maza. 2012. MicroRNA Dysregulation in the Spinal Cord following Traumatic Injury. PLOS ONE 7. Public Library of Science: e34534. [CrossRef]
- Kertesz, Michael, Nicola Iovino, Ulrich Unnerstall, Ulrike Gaul, and Eran Segal. 2007. The role of site accessibility in microRNA target recognition. Nature Genetics 39. Nature Publishing Group: 1278–1284. [CrossRef]
- Liu, Chaochun, Bibekanand Mallick, Dang Long, William A. Rennie, Adam Wolenc, C. Steven Carmack, and Ye Ding. 2013. CLIP-based prediction of mammalian microRNA binding sites. Nucleic Acids Research 41: e138. [CrossRef]
- Kishore, Shivendra, Lukasz Jaskiewicz, Lukas Burger, Jean Hausser, Mohsen Khorshid, and Mihaela Zavolan. 2011. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nature Methods 8: 559–564. [CrossRef]
- Atif, Hamna, and Steven D Hicks. 2019. A Review of MicroRNA Biomarkers in Traumatic Brain Injury. Journal of Experimental Neuroscience 13. SAGE Publications Ltd STM: 1179069519832286. [CrossRef]
- Bhalala, Oneil G., Maya Srikanth, and John A. Kessler. 2013. The emerging roles of microRNAs in CNS injuries. Nature reviews. Neurology 9: 328–339. [CrossRef]
- Lei, Ping, Yaohua Li, Xin Chen, Shuyuan Yang, and Jianning Zhang. 2009. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Research 1284: 191–201. [CrossRef]
- Casha, S., W. R. Yu, and M. G. Fehlings. 2001. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 103: 203–218. [CrossRef]
- Vaccari, Juan Pablo de Rivero, George Lotocki, Alex E. Marcillo, W. Dalton Dietrich, and Robert W. Keane. 2008. A Molecular Platform in Neurons Regulates Inflammation after Spinal Cord Injury. Journal of Neuroscience 28. Society for Neuroscience: 3404–3414. [CrossRef]
- Gao, Lu, Xuehua Pu, Yujing Huang, and Jing Huang. 2019. MicroRNA-340-5p relieved chronic constriction injury-induced neuropathic pain by targeting Rap1A in rat model. Genes & Genomics 41: 713–721. [CrossRef]
- Jiang, Hui, Jie Ni, Yan Zheng, and Yun Xu. 2021. Knockdown of lncRNA SNHG14 alleviates LPS-induced inflammation and apoptosis of PC12 cells by regulating miR-181b-5p. Experimental and Therapeutic Medicine 21: 497. [CrossRef]
- Zhang, Meng, Lin Wang, Sihua Huang, and Xijing He. 2021. Exosomes with high level of miR-181c from bone marrow-derived mesenchymal stem cells inhibit inflammation and apoptosis to alleviate spinal cord injury. Journal of Molecular Histology 52: 301–311. [CrossRef]
- Zhang, Tao, Shuangfei Ni, Zixiang Luo, Ye Lang, Jianzhong Hu, and Hongbin Lu. 2019. The protective effect of microRNA-21 in neurons after spinal cord injury. Spinal Cord 57. Nature Publishing Group: 141–149. [CrossRef]
- 59. Jiang, Xue-Ping, Wen-Bing Ai, Lin-Yan Wan, Yan-Qiong Zhang, and Jiang-Feng Wu. 2017. The roles of microRNA families in hepatic fibrosis. Cell & Bioscience 7. [CrossRef]
- Lagos-Quintana, Mariana, Reinhard Rauhut, Jutta Meyer, Arndt Borkhardt, and Thomas Tuschl. 2003. New microRNAs from mouse and human. RNA (New York, N.Y.) 9: 175–179. [CrossRef]
- Liu, Gang, Megan Ryan Detloff, Kassi N. Miller, Lauren Santi, and John D. Houlé. 2012. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Experimental Neurology 233. Special Issue: Stress and Neurological Disease: 447–456. [CrossRef]
- Tsujimura, Keita, Koichiro Irie, Hideyuki Nakashima, Yoshihiro Egashira, Yoichiro Fukao, Masayuki Fujiwara, Masayuki Itoh, et al. 2015. miR-199a Links MeCP2 with mTOR Signaling and Its Dysregulation Leads to Rett Syndrome Phenotypes. Cell Reports 12: 1887–1901. [CrossRef]
- Landgraf, Pablo, Mirabela Rusu, Robert Sheridan, Alain Sewer, Nicola Iovino, Alexei Aravin, Sébastien Pfeffer, et al. 2007. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129: 1401–1414. [CrossRef]
- Xie, Yili, Lisa A. Tobin, Jordi Camps, Danny Wangsa, Jianhui Yang, Mahadev Rao, Erika Witasp, et al. 2013. MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in cancer cells. Oncogene 32: 2442–2451. [CrossRef]
- Jonas, Stefanie, and Elisa Izaurralde. 2015. Towards a molecular understanding of microRNA-mediated gene silencing. Nature Reviews Genetics 16: 421–433. [CrossRef]
- Li, Feng, Jing Liang, Hua Tong, Shuai Zhu, and Dongfang Tang. 2020. Inhibition of microRNA-199a-5p ameliorates oxygen-glucose deprivation/reoxygenation-induced apoptosis and oxidative stress in HT22 neurons by targeting Brg1 to activate Nrf2/HO-1 signalling. Clinical and Experimental Pharmacology and Physiology 47: 1020–1029. [CrossRef]
- Zhong, Wei, Yong-Chang Li, Qian-Yi Huang, and Xiang-Qi Tang. 2020. lncRNA ANRIL Ameliorates Oxygen and Glucose Deprivation (OGD) Induced Injury in Neuron Cells via miR-199a-5p/CAV-1 Axis. Neurochemical Research 45: 772–782. [CrossRef]
- Zhang, Xianghui, and Guan’en Zhou. 2020. MiR-199a-5p inhibition protects cognitive function of ischemic stroke rats by AKT signaling pathway. American Journal of Translational Research 12: 6549–6558.
- Gao, Zhengchao, Yingjie Zhao, Xijing He, Zikuan Leng, Xiaoqian Zhou, Hui Song, Rui Wang, et al. 2020. Transplantation of sh-miR-199a-5p-Modified Olfactory Ensheathing Cells Promotes the Functional Recovery in Rats with Contusive Spinal Cord Injury. Cell Transplantation 29. SAGE Publications Inc: 0963689720916173. [CrossRef]
- Arneson, Douglas, Guanglin Zhang, Zhe Ying, Yumei Zhuang, Hyae Ran Byun, In Sook Ahn, Fernando Gomez-Pinilla, and Xia Yang. 2018. Single cell molecular alterations reveal target cells and pathways of concussive brain injury. Nature communications 9: 3894. [CrossRef]
- Xu, Guanghui, Rongguang Ao, Zhongzheng Zhi, Jianbo Jia, and Baoqing Yu. 2019. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. Journal of Cellular Physiology 234: 10205–10217. [CrossRef]
- Kang, Jian, Zhenhuan Li, Zhongzheng Zhi, Shiqiang Wang, and Guanghui Xu. 2019. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Therapy 26: 491–503. [CrossRef]
- Zhang, Zhong, Jian Wang, Zongbin Song, Yunjiao Wang, Zhigang Cheng, Qulian Guo, E. Wang, Yanping Jian, and Lei Wu. 2021. Downregulation of microRNA-199a-5p alleviated lidocaine-induced sensory dysfunction and spinal cord myelin lesions in a rat model. Toxicology Letters 336: 1–10. [CrossRef]
- Zhou, Qian, Ming-Ming Zhang, Min Liu, Zhi-Gang Tan, Qi-Lin Qin, and Yu-Gang Jiang. 2021. LncRNA XIST sponges miR-199a-3p to modulate the Sp1/LRRK2 signal pathway to accelerate Parkinson’s disease progression. Aging (Albany NY) 13: 4115–4137. [CrossRef]
- Bao, Ning, Bo Fang, Huangwei Lv, Yanhua Jiang, Fengshou Chen, Zhilin Wang, and Hong Ma. 2018. Upregulation of miR-199a-5p Protects Spinal Cord Against Ischemia/Reperfusion-Induced Injury via Downregulation of ECE1 in Rat. Cellular and Molecular Neurobiology 38: 1293–1303. [CrossRef]
- Strickland, E. R., M. A. Hook, S. Balaraman, J. R. Huie, J. W. Grau, and R. C. Miranda. 2011. MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neuroscience 186: 146–160. [CrossRef]
- Lim, Lee P., Nelson C. Lau, Earl G. Weinstein, Aliaa Abdelhakim, Soraya Yekta, Matthew W. Rhoades, Christopher B. Burge, and David P. Bartel. 2003. The microRNAs of Caenorhabditis elegans. Genes & Development 17: 991–1008. [CrossRef]
- Alexiou, Panagiotis, Manolis Maragkakis, Giorgos L. Papadopoulos, Martin Reczko, and Artemis G. Hatzigeorgiou. 2009. Lost in translation: an assessment and perspective for computational microRNA target identification. Bioinformatics 25: 3049–3055. [CrossRef]
- Witkos, T. M., E. Koscianska, and W. J. Krzyzosiak. 2011. Practical Aspects of microRNA Target Prediction. Current Molecular Medicine 11: 93–109. [CrossRef]
- Zuker, Michael. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research 31: 3406–3415. [CrossRef]
- STarMir: a web server for prediction of microRNA binding sites | Nucleic Acids Research | Oxford Academic. 2022. https://academic.oup.com/nar/article/42/W1/W114/2436353. Accessed December 19.
- Basso, D. M., M. S. Beattie, and J. C. Bresnahan. 1995. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma 12: 1–21. [CrossRef]
- Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses | SpringerLink. 2022. https://link.springer.com/article/10.3758/BRM.41.4.1149. Accessed September 28.
- Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 25: 402–408. [CrossRef]
- Headrick, Todd C. 2009. Statistical Simulation: Power Method Polynomials and Other Transformations. New York: Chapman and Hall/CRC. [CrossRef]
- Schneider, Caroline A., Wayne S. Rasband, and Kevin W. Eliceiri. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9. Nature Publishing Group: 671–675. [CrossRef]
- Bankhead, Peter, Maurice B. Loughrey, José A. Fernández, Yvonne Dombrowski, Darragh G. McArt, Philip D. Dunne, Stephen McQuaid, et al. 2017. QuPath: Open source software for digital pathology image analysis. Scientific Reports 7. Nature Publishing Group: 16878. [CrossRef]
- Søe, Martin J., Trine Møller, Martin Dufva, and Kim Holmstrøm. 2011. A Sensitive Alternative for MicroRNA In Situ Hybridizations Using Probes of 2′-O-Methyl RNA + LNA. Journal of Histochemistry and Cytochemistry 59: 661–672. [CrossRef]
| Primer | Sequences (5’-3’) |
| XIAP 3′UTR-wt |
Forward: ATCGAGCTCCACAGTAGGCATGTTATG Reverse: ATAGTCGACCTGTGATGCTTTTCTATGTCAG |
| XIAP 3’UTR-mut |
Forward: GTTCCAAGATCTTTGGAGG Reverse: CCTCCAAAGATCTTGGAACAGTTC |
| pmiRGLO sequencing | CAAGAAGGGCGGCAAGATCG |
| Antibody | Reference |
| Primary antibodies for Immunoblot | |
| anti-Actin | BD Biosciences Cat# 612656, RRID:AB_2289199 |
| anti-XIAP | R and D Systems Cat# AF8221, RRID:AB_2215008 |
| Primary antibodies for Immunofluorescence | |
| anti-XIAP | R and D Systems Cat# AF8221, RRID:AB_2215008Abcam Cat# ab21278, RRID:AB_446157 |
| anti-NeuN | Millipore Cat# ABN78, RRID:AB_10807945 |
| Primary antibodies for FISH | |
| Alkaline phosphatase-conjugated sheep-anti-digoxigenin | Sigma-Aldrich Cat# 11093274910, RRID:AB_2734716 |
| Secondary antibodies for Immunobloting | |
| HRP-conjugated goat anti-rabbit | Cell Signaling Technology Cat# 7074, RRID:AB_2099233 |
| HRP-conjugated goat anti-mouse | Cell Signaling Technology Cat# 7076, RRID:AB_330924 |
| Secondary antibodies for Immunofluorescence | |
| Alexa Fluor 488 goat anti-rabbit | Life Technologies Cat# A11034, RRID:AB_10562715 |
| Alexa Fluor 488 goat anti-mouse highly-cross adsorbed | Molecular Probes Cat# A11029, RRID:AB_138404 |
| Probe | Sequence |
| Negative control | 5'- DIG - [G]{T}[GU]{AC]{A}[CG]{T}[CU]{A}[UA]{C}[GC]{C}[C}[CA] - 3’ |
| miR-199a-5p | 5'-DIG - {G}[AA]{C}[AG]{G}[UA]{G}[UC]{T}[GA]{A}[CA]{C}[UG]{GG} - 3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





