Introduction
Diabetes mellitus is a complex immune disease characterized by systemic dysregulation of glucolipid metabolism and chronic, low-grade inflammation caused by hyperglycemia1 . Pyroptosis is a pro-inflammatory mode of programmed cell death in which the activation of pore-forming protein-D (GSDMD) by NLPR3 inflammatory vesicles induces the formation of pores in the cytosol, leading to cell swelling and rapid lysis of cell contents and the release of several pro-inflammatory mediators such as interleukin-1β (IL-1β) and interleukin-18 (IL-18)2 . It has now been shown that scorch death is involved in the inflammatory response and can promote the progression of diabetes and its multiple complications3,4.Some molecular proteins targeting pyroptosis and related signaling pathways may be a new potential target for the management and treatment of diabetes and its complications. In this study, a comprehensive quantitative and visual analysis of the literature related to diabetes and pyroptosis research was carried out through bibliometric analysis with the help of VOSviewer and other related professional literature analysis software. In addition, for clinicians and scholars studying diabetes, its complications and pyroptosis, the results of this study will not only provide information on the hot spots and dynamic progress of research in this field, but also provide important research directions.
Information and Methodology
Data: from the Web of science database (http: //web of science.com), search method: combination of subject and free word search, time span from 1985 to 2022, type of literature is thesis, language is English, search time set to August 13, 2022.
1. The search formula is as follows.
#1 TOPIC:(diabetes mellitus) OR TOPIC:(diabetes) OR TOPIC:(diabetic) OR TOPIC:(glycosuria) OR TOPIC:(glycuresis) OR TOPIC:(Diabetes Mellitus, Type 1 ) OR TOPIC:(T1DM) OR TOPIC:( Diabetes Mellitus, Type 2 ) OR TOPIC:(T2DM)
#2 TOPIC:( Pyroptosis ) OR TOPIC:(Pyroptoses) OR TOPIC:(Cell Deaths, Pyroptotic) OR TOPIC:(Cell Deaths, Pyroptotic) OR TOPIC:(Deaths, Pyroptotic Cell) OR TOPIC:(Deaths, Pyroptotic Cell ) OR TOPIC:(Deaths, Pyroptotic Cell) OR TOPIC:(Pyroptotic Cell Deaths) OR TOPIC:(Caspase-1 Dependent Cell Death) OR TOPIC:(Caspase 1 Dependent Cell Death) OR TOPIC:(Inflammatory Apoptosis) OR TOPIC:(Apoptoses, Inflammatory) OR TOPIC:(Apoptosis, Inflammatory) OR TOPIC:(Inflammatory Apoptoses)
#3 #1 AND #2 , Indexes=SCI- EXPANDED, SSCI, Timespan=1985 - 2022
2. Inclusion and exclusion criteria
2.1 Inclusion criteria: Published research-based English-language thesis literature relevant to the study of diabetes and pyroptosis was met.
2.2 Exclusion criteria: Reviews, Narrative Reviews, Guidelines, Consensus, and Literature with incomplete information.
3. Methods: Select the literature type as thesis and after that update the search page to get the updated search results. After selecting all the results, the record content was selected as full record for export and the export format was txt format. Statistical collation and visual analysis of the included literature data package was performed through VOSviewer 1.6.18 freeware and Microsoft Excel 2019 (no license required)5 . The main information extracted included authors, year of publication, journal of publication, number of citations, scientific institution, country or region, keywords and other important literature data, and the research topics were identified through the titles, keywords and abstracts of the literature and, if necessary, through full-text reading analysis. All the search and inclusion process was done independently by two researchers, and the literature or data with disagreement was then independently confirmed by a third researcher.
Results
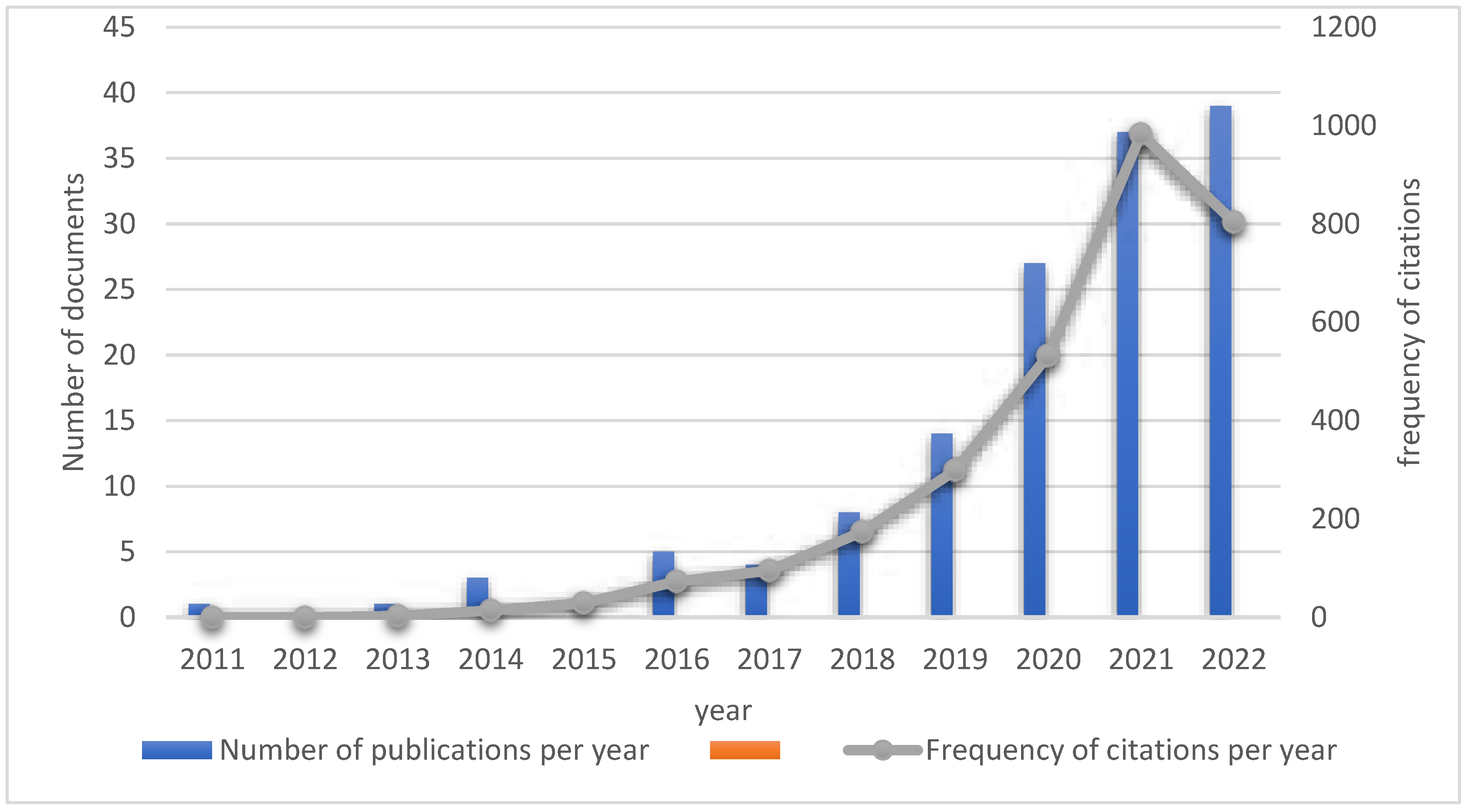
1. Distribution of literature publication time: The literature search revealed that there was no published literature on diabetes and pyroptosis related studies between 1985 and 2011. From 2011 to 2022, a total of 139 publications were searched in this field, with an average of 11.6 publications per year, of which no publications were retrieved in 2012 and 2015, and the number of publications increased rapidly from 2018 onwards, with 39 publications in 2022 as of August (
Figure 1).
2. Number of citations per year of published literature : from 2011 to 2022, 139 published literature were cited 3009 times, 2877 times after removing self-citations, with a mean H-index of 27 (Figure 1-1). The top citation frequency for a single published literature was by an author from GermanySchmid-Burgk, JL
6 In addition, 8 of the top 10 cited articles were from China, as shown below (
Table 1).
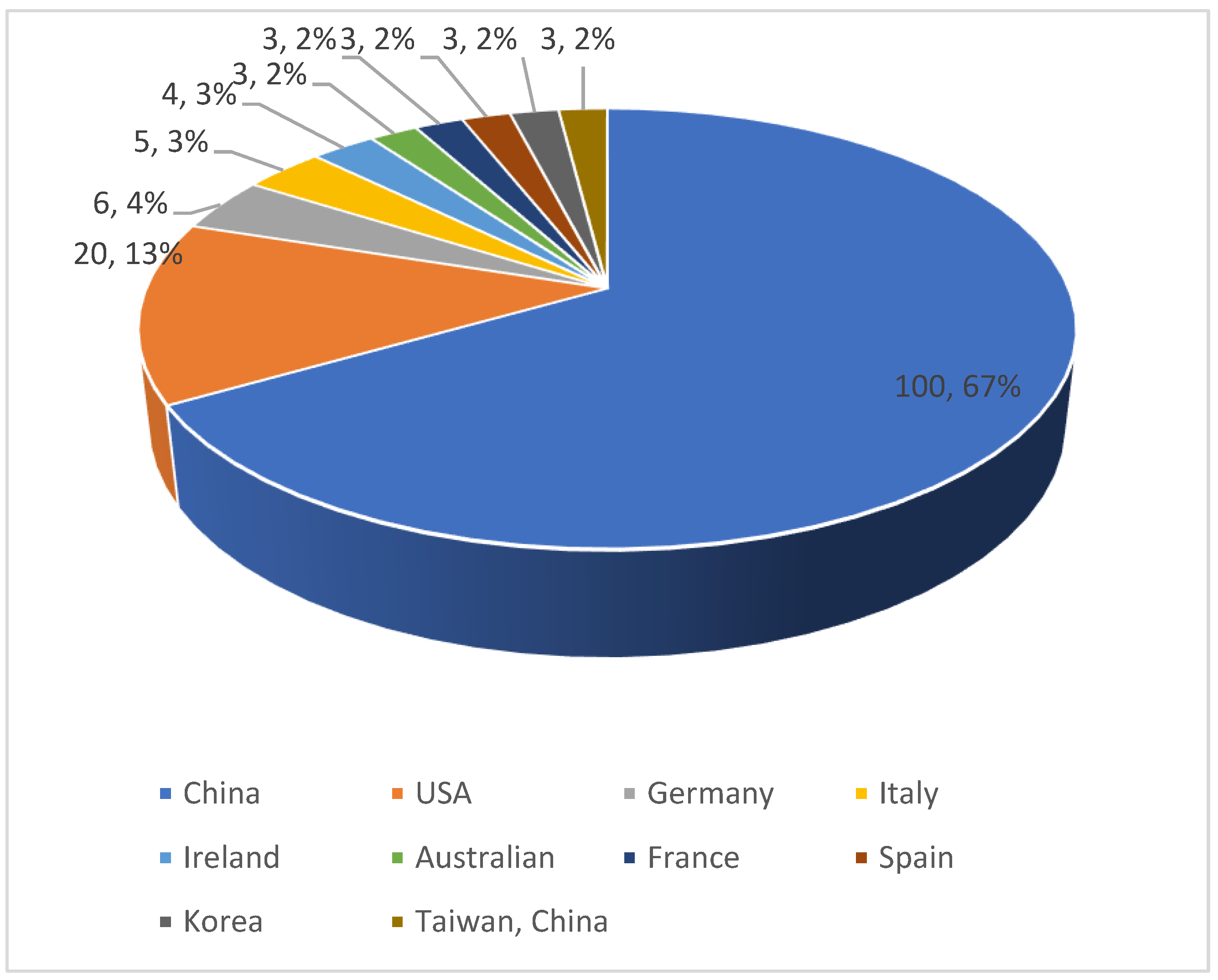
3. Number of publications in different countries/regions: In 2011-2022, a total of 20 countries/regions published literature related to diabetes and pyroptosis research, and China has accumulated 100 publications so far, accounting for 67%, ranking first, followed by the United States (20, 13%), Germany (6, 4%), Italy (5, 3%). The volume of publications from other countries is shown in
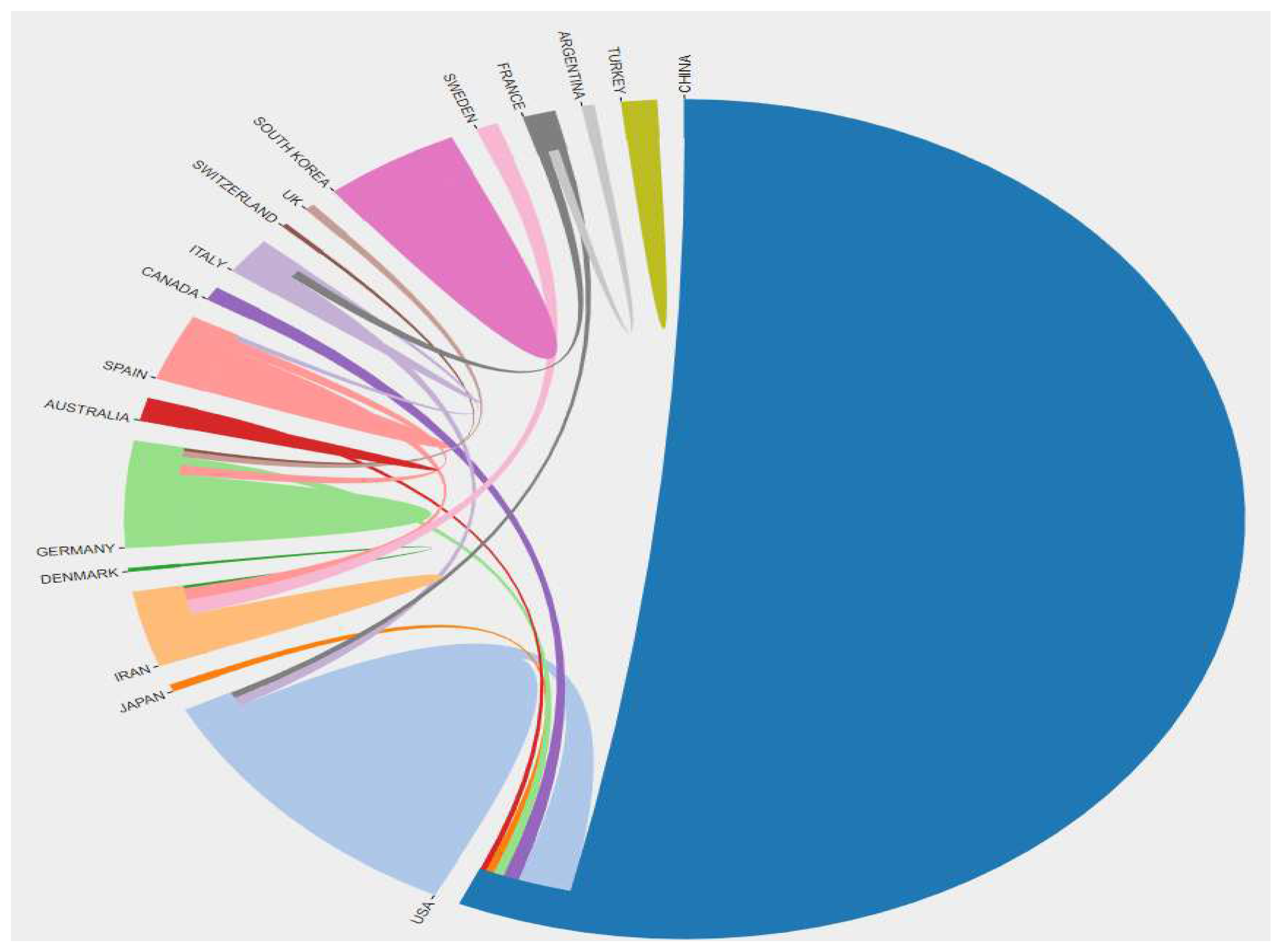
Figure 2. At the same time, there is a close cooperation between these publishing countries/regions, as shown in
Figure 3.
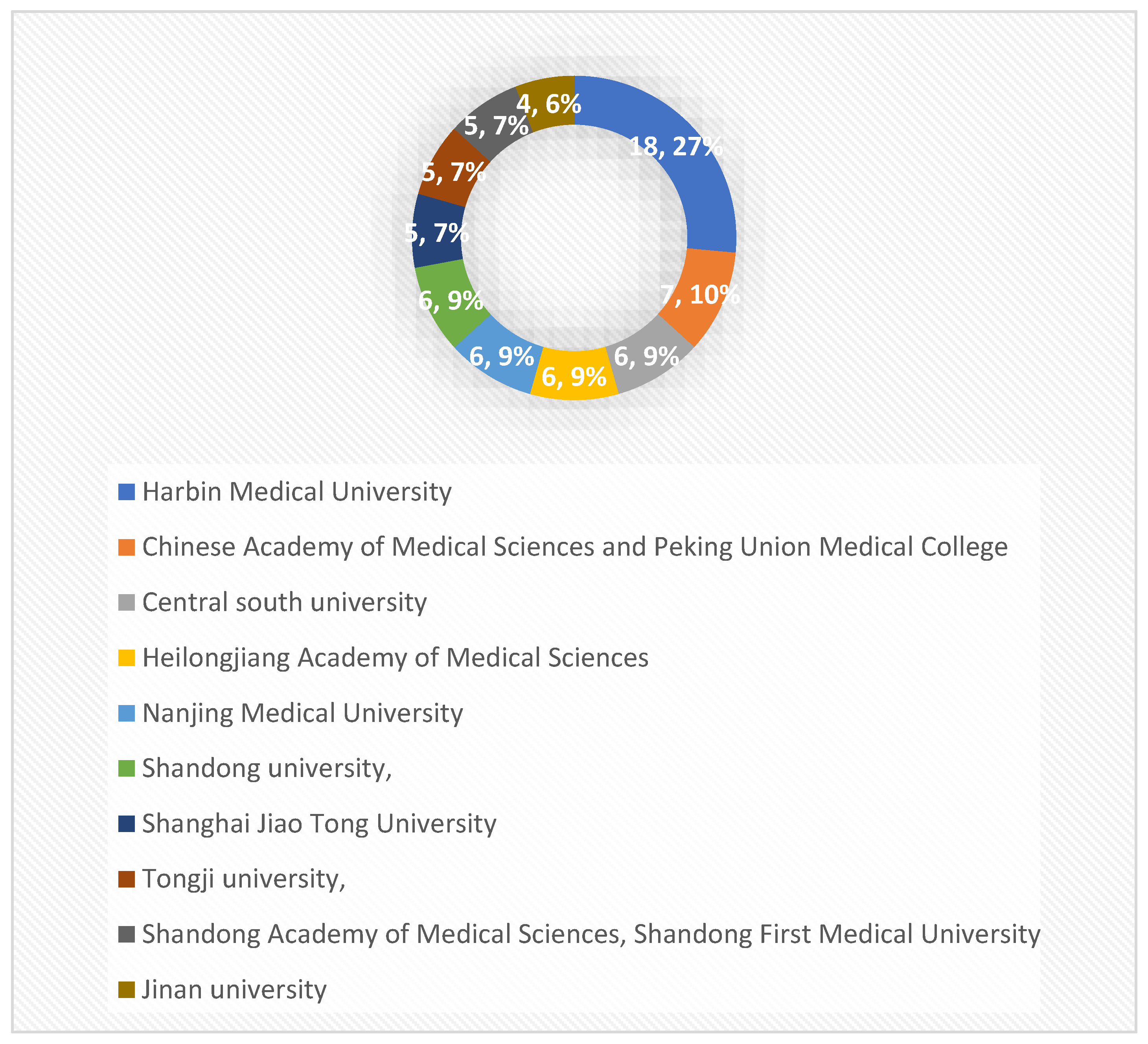
4. The number of publications and citations of different research institutions: the 139 publications from 2011-2022 involved 222 relevant universities and other research institutions, among which, Harbin Medical University ranked first with 18 publications; Peking Union Medical College of the Chinese Academy of Medical Sciences ranked second with 7 publications; the top 10 research institutions with the most publications were all from China (
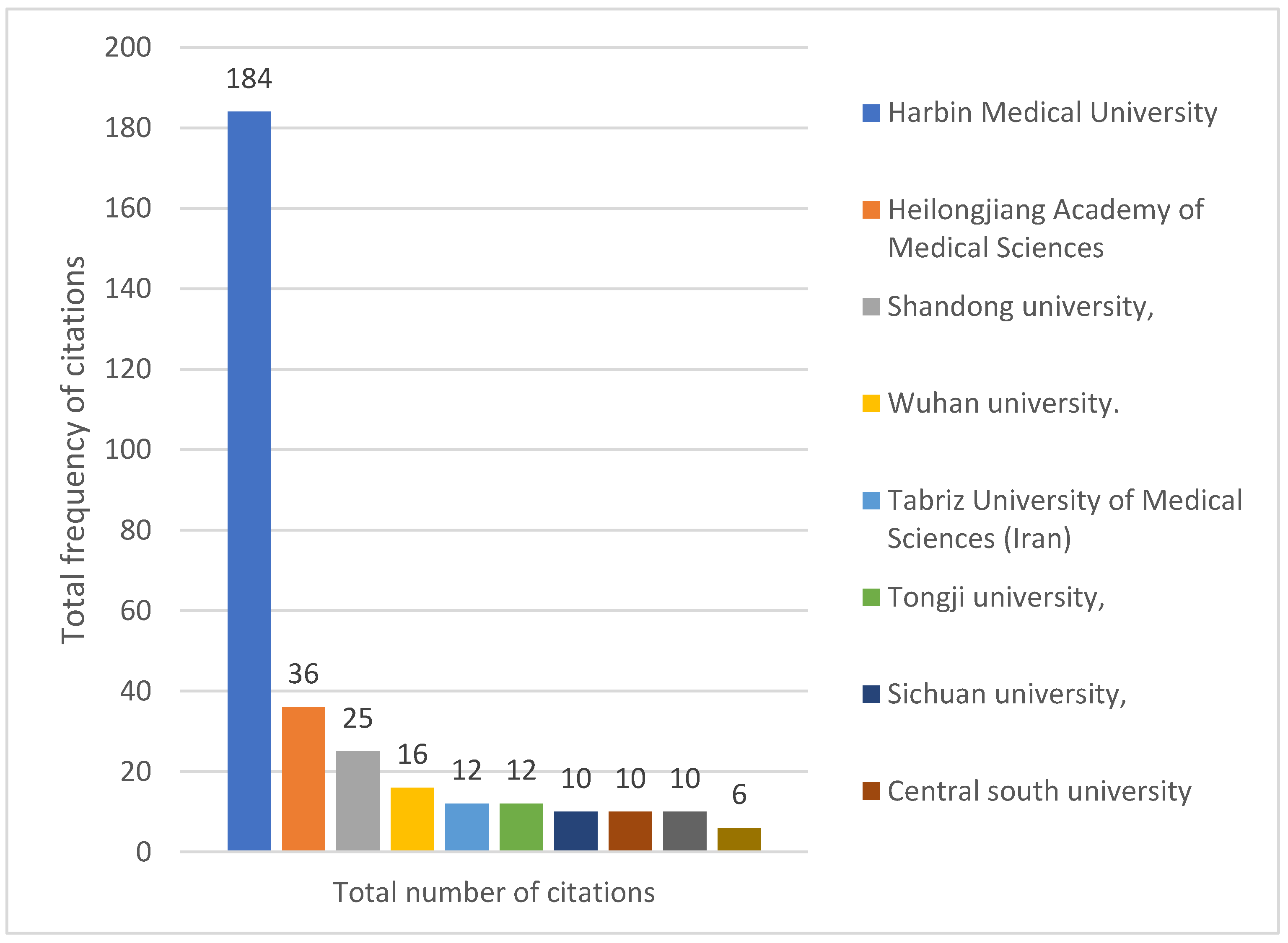
Figure 4). In addition, from the analysis of the citations of the published literature of research institutions, Harbin Medical University is the most influential research institution in this field with 184 total citations, followed by domestic and foreign university research institutions such as Heilongjiang Academy of Medical Sciences (36 total citations) and Shandong University (25 total citations) (
Figure 5), as shown in
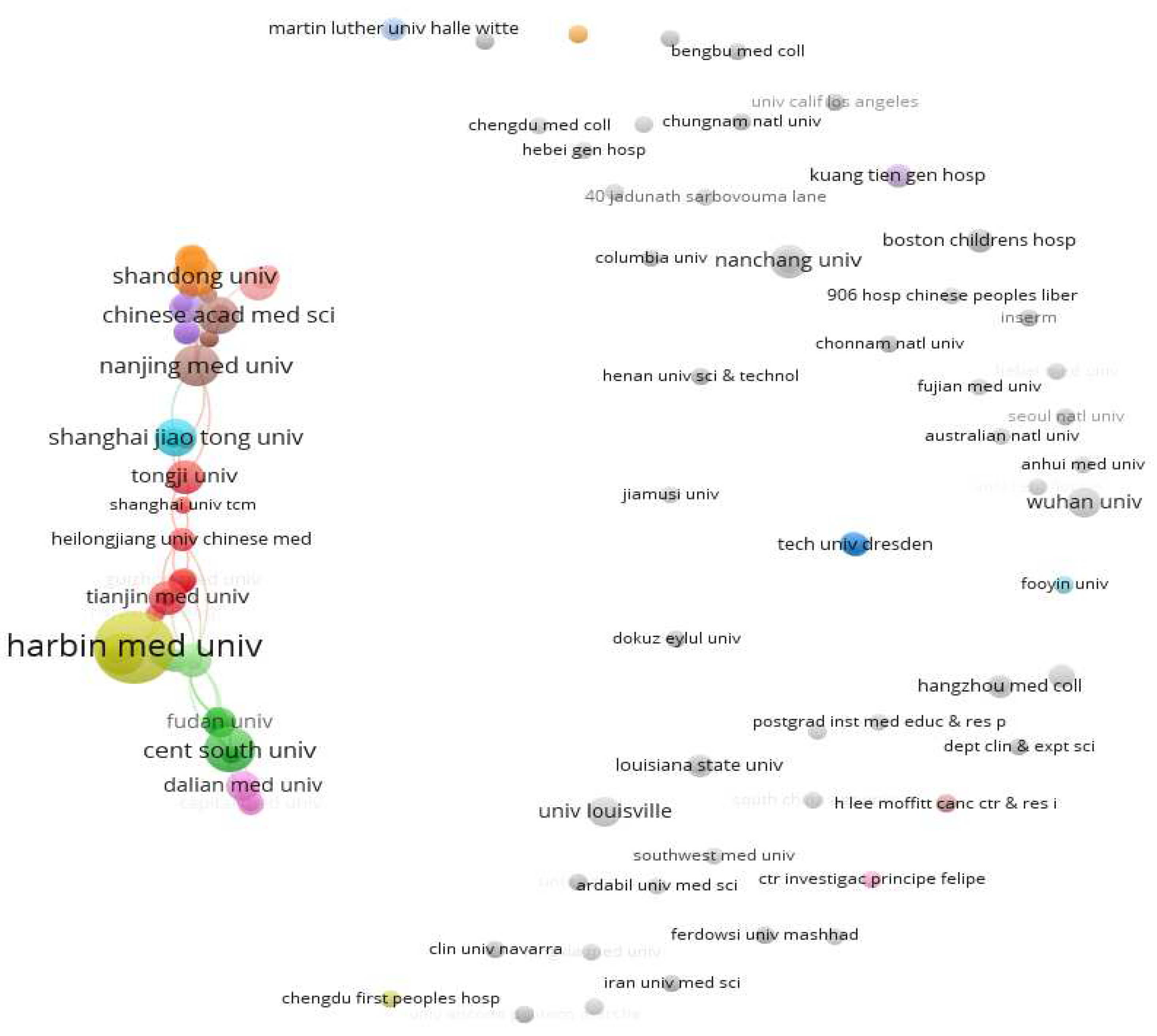
Figure 6, there are also close academic exchanges and cooperation among these research institutions relationship.
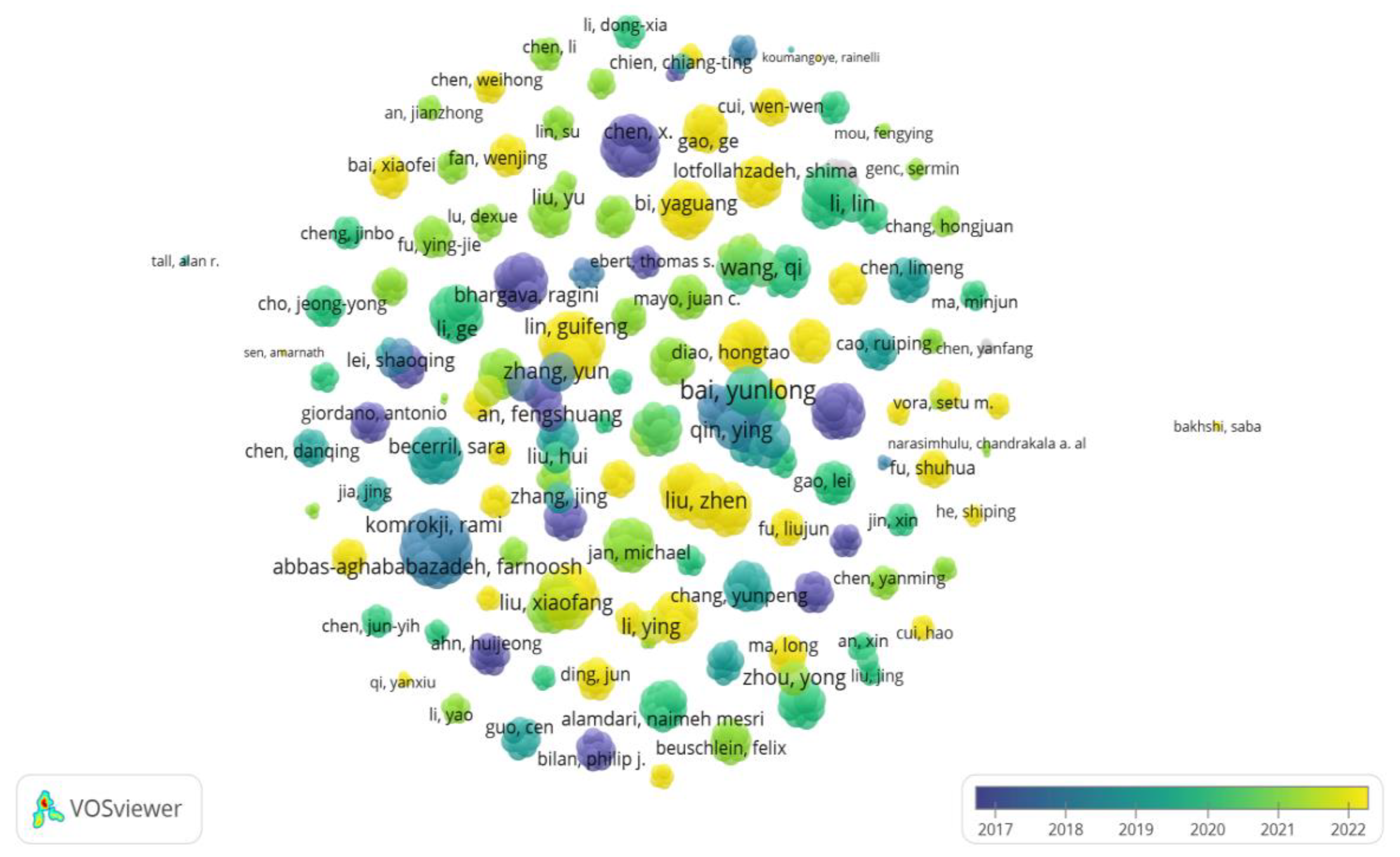
5. The number of publications and citations by different authors: a total of 980 authors collaborated with each other to publish 139 papers related to the field of diabetes and pyroptosis from 2011-2022, and the top 10 influential authors are shown in
Table 2, with author Chen, X (5 publications, 29 total citations) ranking first in terms of influence, followed by Wang, Y Q, Che, H, and Wang, L H (4 publications, 29 total citations); in addition, the collaboration between different authors is shown in Figure 7.
6. Number of literature published in different journals: 139 papers included in this study from 2011-2022 were published in 98 different journals and magazines, respectively. Based on the data of journal volume and highly cited information, "CELL DEATH DISEASE" is the top journal in both volume and impact in the field of diabetes and pyroptosis, with Chinese Academy of Sciences (CAS) region I, impact factor 9.685, with an H-index of 59; in addition, the top 10 journals in terms of number of articles and high citations are shown in
Table 3 and
Table 4, respectively.
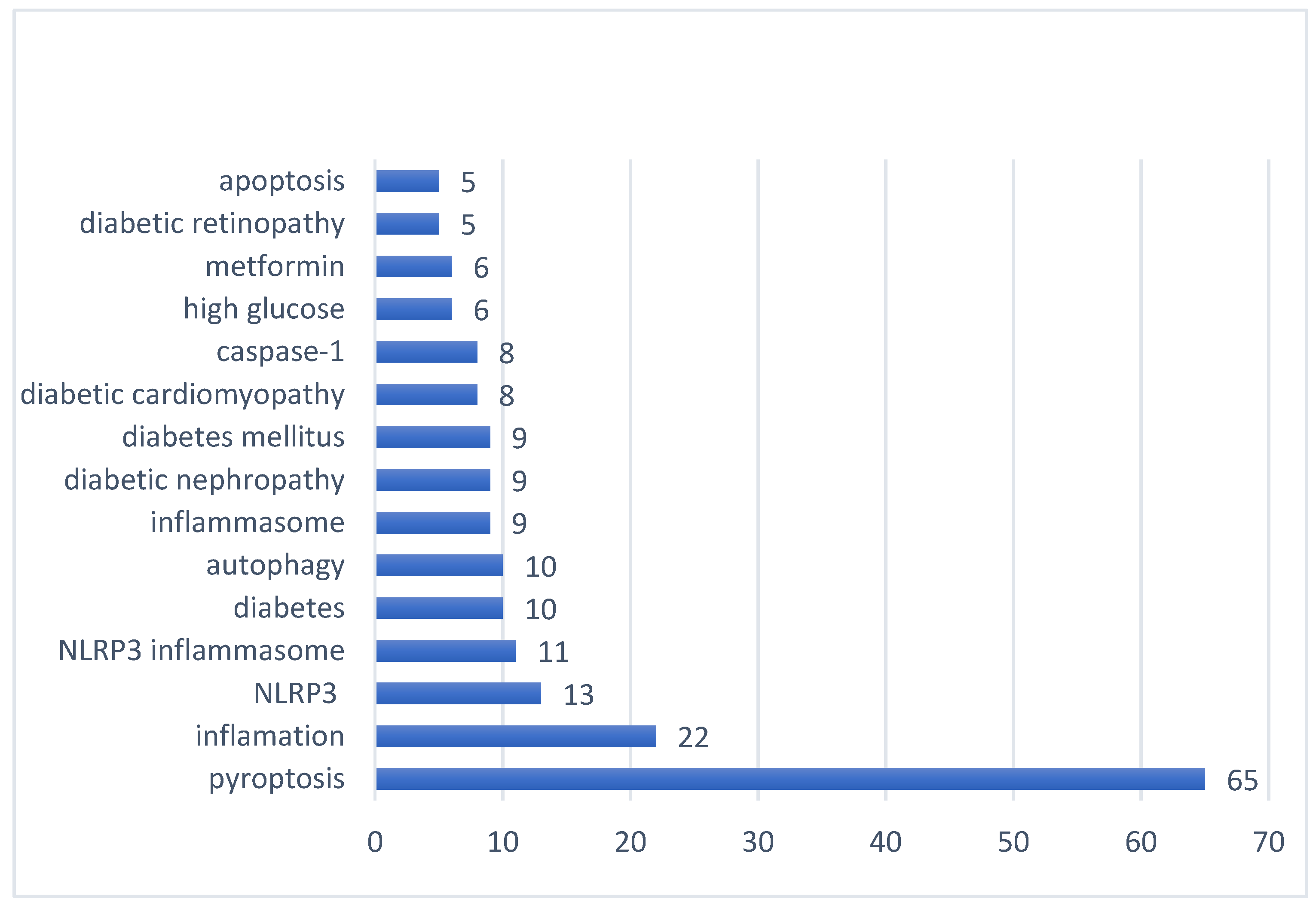
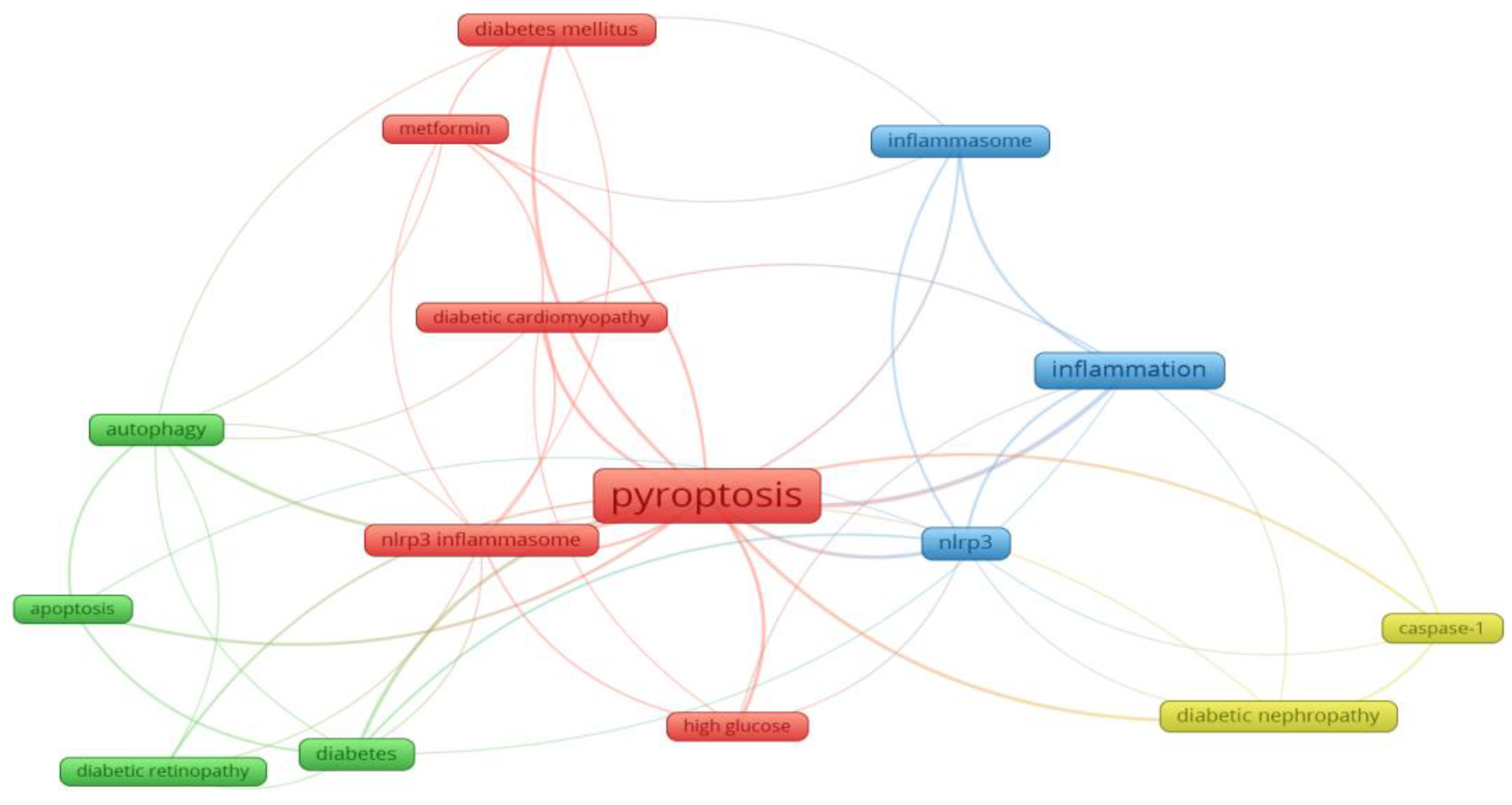
7. Analysis of research hotspots: The frequency of keywords in the published literature can be used to analyze the current hotspots in the research field. From 139 papers published in 2011-2022, 349 keywords can be extracted, the first keyword "cell death" has a cumulative frequency of 65, the second and third keywords are "inflammation" and "NLRP3" respectively. and the other top 15 high-frequency keywords are shown in
Figure 8. In addition, as shown in
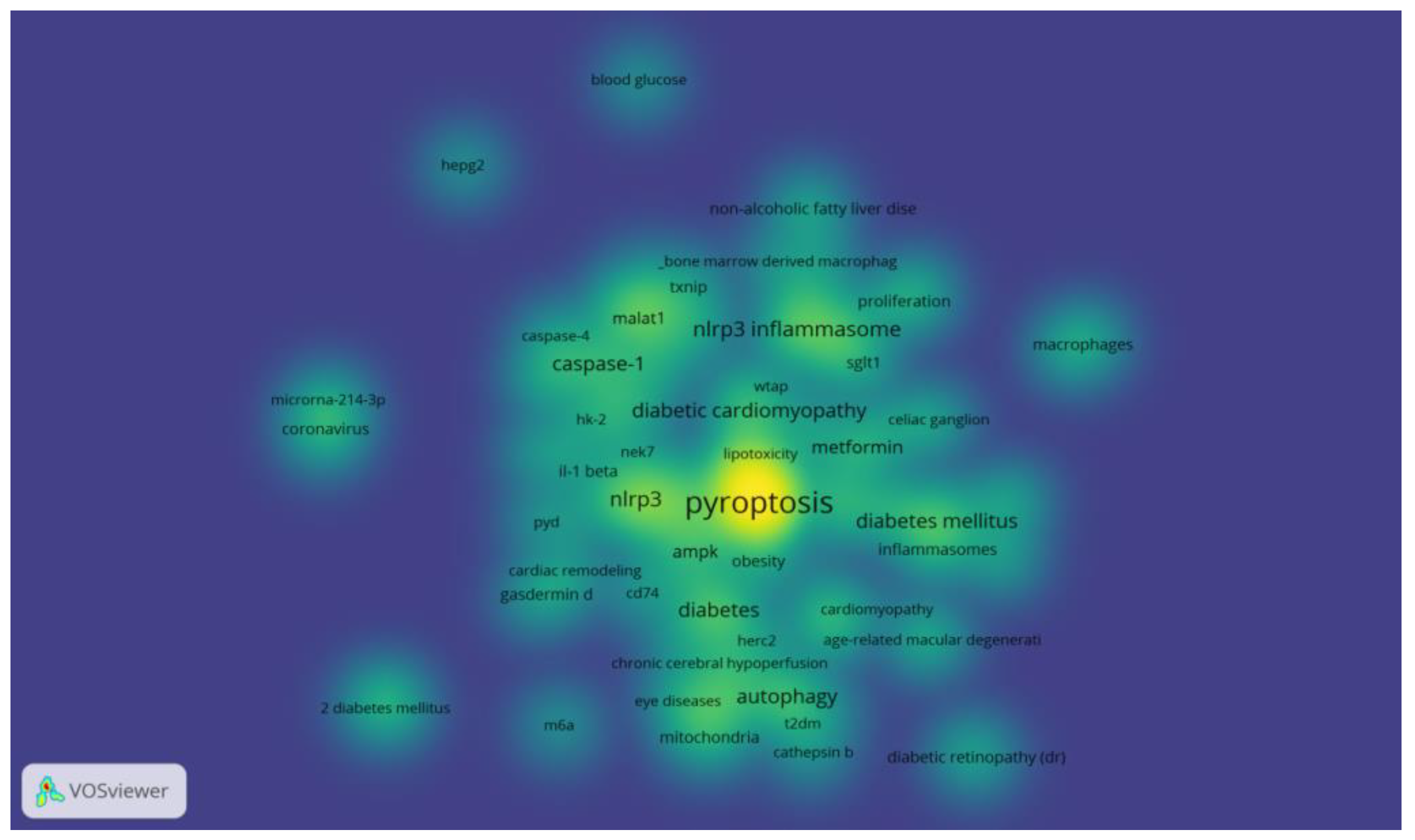
Figure 9, the clustering network analysis of high-frequency keywords by VOSviewer revealed that the key proteins of pyroptosis, "NLRP3", "inflammatory vesicles", "NLRP3 inflammatory vesicles" and "caspase-1" centered on "pyroptosis", while "autophagy" and "apoptosis" were involved. "The only relevant therapeutic drug is "metformin" among the high-frequency keywords, which is involved in the development of hyperglycemia, diabetes and its chronic complications DR, DN and DCM. The density distribution of keywords in the published literature in the field of diabetes and pyroptosis is shown in
Figure 10.
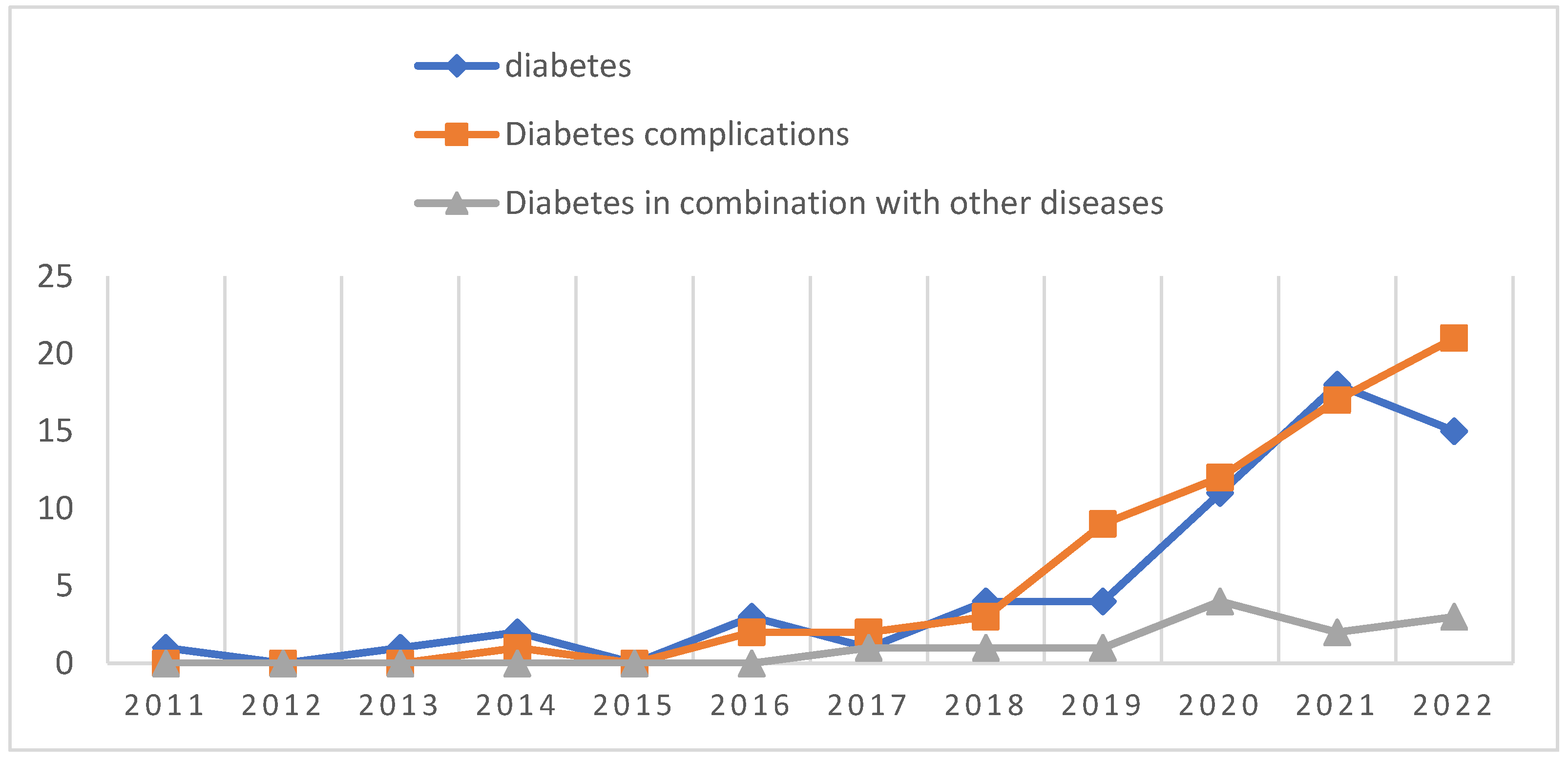
8. Analysis of the dynamics of research themes: by thematically locating and classifying the literature included in this study, it was found that the largest number of literature (67) studied the association of pyroptosis with diabetic complications, including DCM (27), DN (17) and DR (10), followed by literature (60) that studied the association of pyroptosis with the pathogenesis and therapeutic targets of diabetes, and finally, literature related to diabetes combined with Lastly, the literature related to other diseases (12). From the analysis of the timeline of literature publication, 2 papers published in 2011 and 2013 were on the topic of diabetes and pyroptosis, 1 paper was published in 2014 on the topic of diabetic complications, no literature published in 2012 and 2015 on the above related topics were inquired, 1 paper was published in 2017 on the topic of diabetes combined with other diseases, and before 2019 The number of publications on all 3 of the above topics was low, and then the number of publications on diabetes and its complications with pyroptosis studies increased year by year, especially the rate of publications on diabetes complications with pyroptosis studies increased faster (
Figure 11).
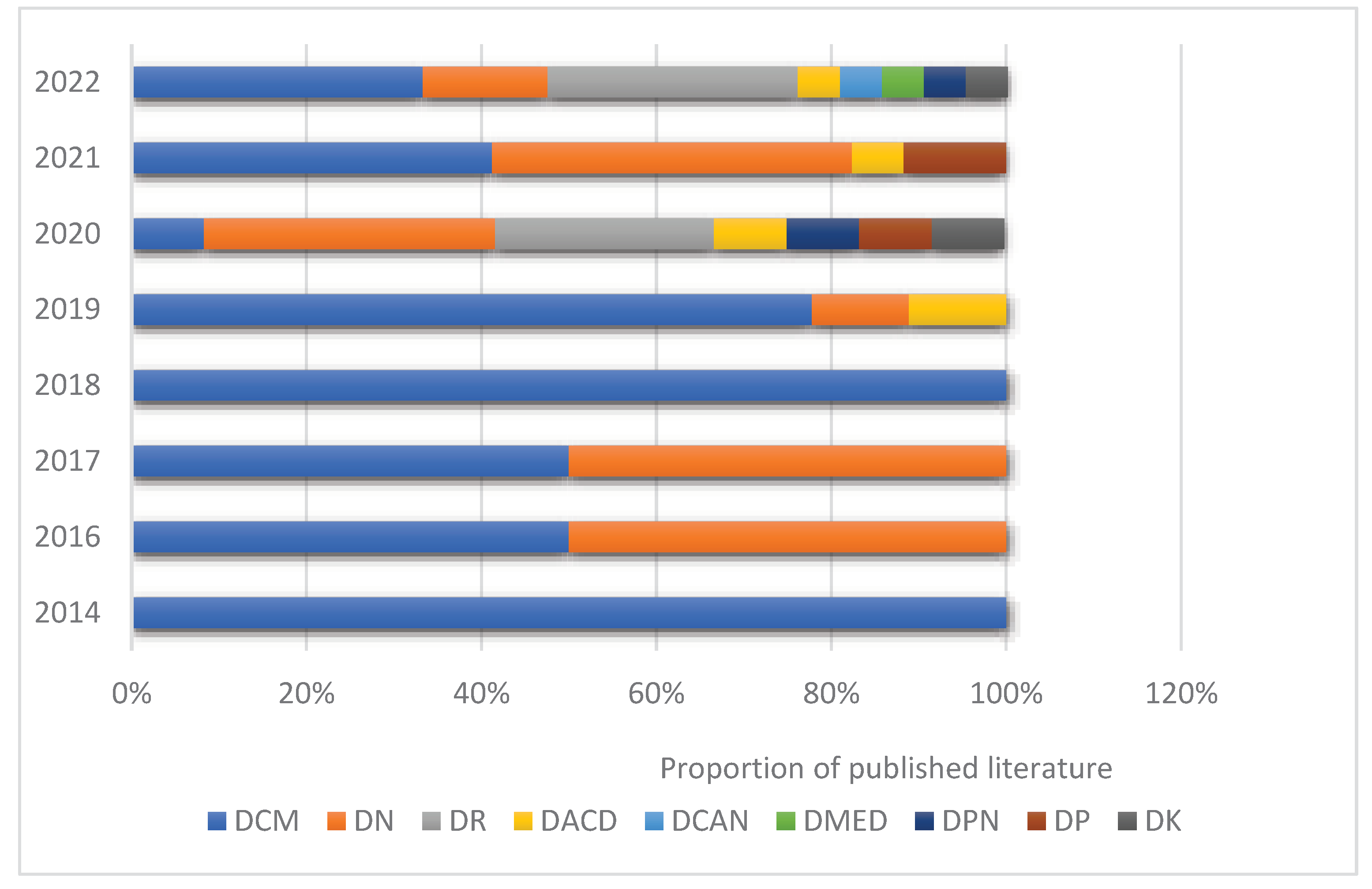
The first literature studying diabetic cardiomyopathy and pyroptosis was published in 2014, which was also the first literature related to diabetic complications and pyroptosis until 2019, the number of published literature studying diabetic cardiomyopathy ranked first, followed by diabetic nephropathy and diabetic cognitive dysfunction. From 2020, the number of published literature on diabetic complications and pyroptosis studies has increased rapidly, and there has been a gradual increase in published literature on different types of diabetic complications, such as diabetic retinopathy, diabetic keratopathy, and diabetic periodontitis. From 2014 to the present, diabetic cardiomyopathy and diabetic nephropathy have ranked first in terms of the percentage of published literature per year, in addition to 2020 The proportion of the annual number of published literature for diabetic retinopathy has increased rapidly and has ranked second by 2022 (
Figure 12).
Discussions
From 2011-2022, during this past approximately 12 years, diabetes and pyroptosis have made progress in areas related to research on pathogenesis, signaling pathways, and therapeutic targets, which have far-reaching implications for future diabetes-related basic research and clinical treatment strategies. This study provides a comprehensive, quantitative and visual analysis of 139 papers related to diabetes and pyroptosis published in the past 12 years based on bibliometrics. With the rapid development of the new era and the increasing trend of globalization, such as scientific and technological research, the research articles included in this study were completed by researchers collaborating with each other among research institutions in different countries or regions. From the analysis of the timeline, the number of published literature on diabetes and pyroptosis has been increasing year by year, especially in the past three years, although no relevant literature was retrieved in 2012 and 2015, which indicates that although the research on diabetes and pyroptosis started late, it has developed more rapidly and received more and more attention in recent years. In terms of research countries or regions, China and the United States are far ahead of other countries or regions in terms of both the number of publications per year and the cumulative number of publications, which indicates that scholars from China and the United States have done a lot of research work in this field. Further analysis revealed that in the field of diabetes and cell death, the research institution with the highest cumulative number of publications and the highest influence is Harbin Medical University in China, and the top 10 research institutions in terms of cumulative number of publications are all from Chinese universities, and 7 of the top 10 research institutions in terms of influence are from domestic universities, while the rest are from Iranian, Italian and American universities. These data show that Chinese universities have made outstanding contributions in this field, and they also show that universities in China and abroad are important institutions to promote the progress of research in this field. In addition, the first authors of 8 of the top 10 highly cited papers on diabetes and pyroptosis are also from China, and the top 10 highly cited authors of published papers in this field are also Chinese scholars, which indicates that the quality level of Chinese scholars' research in this field has been recognized internationally.
Dabetes and Pyroptosis
The results of high-frequency keyword analysis in this study show the direction of diabetes and pyroptosis research in recent years, mainly around the link between the specific mechanisms by which pyroptosis occurs as an inflammatory response and the pathogenesis and potential therapeutic targets of diabetes. The classical pyroptosis pathway consists of activation of caspase 1 by activated NLPR3 inflammatory vesicles to cleave GSDMD and then form membrane pores in the cell membrane, followed by the efflux of intracellular contents and release of a large number of inflammatory factors such as IL-1β and IL-1816,17 The non-classical pyroptosis pathway is a pathway predicated on the activation of Caspase-4/5/1118 . The important pathological features of diabetes are insulin resistance and pancreatic β-cell destruction leading to impaired insulin secretion, and previous studies have shown a close relationship between the pathogenesis of diabetes and the chronic inflammatory response19 Carlos et al20 demonstrated that mitochondrial DNA activates NLPR3 inflammatory vesicles and releases the pro-inflammatory factor IL-1β to induce type 1 diabetes. In a hyperglycemic environment, reactive oxygen species (ROS) activate NLRP3 inflammatory vesicles in pancreatic β-cells, promoting IL-1β secretion leading to insulin secretion dysfunction, promoting obesity and insulin resistance, and ultimately inducing type 2 diabetes raw21. Palmitic acid (PA) and lipopolysaccharide (LPS)-treated HepG2 cells induce NLRP3 inflammatory vesicles to activate caspase-1 and produce excessive pro-inflammatory cytokines such as IL-1β, IL-18 and TNF-α, impairing the action of insulin receptors, thereby blocking downstream signaling pathways and exacerbating insulin resistance in HepG2 cells. Furthermore, human umbilical cord-derived mesenchymal stem cells (UC-MSCs) co-cultured with HepG2 could effectively alleviate PA and LPS-induced insulin resistance by blocking NLRP3 inflammatory vesicle activation and inflammatory factors. In addition, downregulation of NLRP3 or IL-1β expression partially ameliorated impaired insulin signaling in UC-MSCs. Similarly, UC-MSCs significantly improved hyperglycemia and reduced inflammatory factor activity in type 2 diabetic rats, thereby improving insulin resistance22 . Hypoxia and islet inflammation are involved in pancreatic β-cell failure in type 2 diabetes, and a recent study23 showed that activation of NLRP3 inflammatory vesicles in mouse insulinoma cells under hypoxic conditions leads to an inflammatory response and death of pancreatic β cells and upregulation of ROS and thioredoxin-interacting protein (TXNIP), and finally, TXNIP knockdown experiments in mouse insulinoma cells pretreated with the ROS inhibitor N-acetylcysteine (NAC) revealed that activation of ROS/TXNIP/NLRP3 axis is involved in hypoxia-induced inflammatory responses and cell death in pancreatic β-cells23 . Tripartite motif-containing (TRIM) family proteins as regulators are involved in both autophagy and pyroptosis, while they play critical roles in diabetes mellitus and its complications24.TRIM2725、TRIM3226、TRIM727、TRIM7228、TRIM1329 and TRIM6330 acting as regulatory proteins are involved in the development of DM as well as diabetic complications.The above studies suggest that pyroptosis is emerging as a new research perspective in the pathogenesis and treatment of diabetes. However, there are fewer studies on diabetes and pyroptosis. Whether scorched cell death of pancreatic cells leads to disorders of insulin secretion, especially in trials related to pancreatic β-cell function, needs to be further investigated.
The results of the progress of this research theme show that the number of published studies on diabetic complications and pyroptosis is increasing year by year, especially the study of DCM, DN and DR, the three major chronic complications of diabetes in pyroptosis, is gradually becoming a current hot spot.
Diabetic Cardiomyopathy and Pyroptosis
DCM is a chronic complication of diabetes, characterized by myocardial fibrosis, left ventricular hypertrophy, impaired systolic and diastolic function, which can lead to heart failure and is a key cause of death in diabetic patients31 . Chronic inflammation and fibrosis of the myocardium are important pathological changes in DCM32 . An increasing number of studies have shown that activation of NLRP3 inflammatory vesicles in cardiomyocytes is involved in the development of DCM33 . The Chinese medicine gibberellin can reduce the activation of NLRP3 inflammatory vesicles by inhibiting the production of ROS, thus ameliorating the high glucose-induced myocardial injury34 . Compound Pearl Lipid Regulating Formula is an herbal preparation used clinically to treat disorders of glucolipid metabolism, and a recent study found that it could inhibit DCM by suppressing cardiac lipotoxicity-induced oxidative stress and NLRP3 inflammatory vesicle activation35 . Santosh K et al36 found that hyperglycemia upregulates oxidative stress-induced cell death via apoptosis and pyroptosis in human Cardiac Stem Cells(hCSCs), which is mediated by MMP9. Absence/knockdown of MMP9 improves viability of hCSCs by decreasing oxidative stress and suppressing downstream cell death signaling via apoptosis and pyroptosis,These results suggest that MMP9 may be an effective molecular target to interfere with the progression of DCM.More progress has been made in the study of signaling pathways related to DCM pyroptosis, including TLR4/NF-kB/NLRP3 inflammatory vesicle signaling pathway37,38, AMPK/ROS/TXNIP/NLRP3 inflammatory vesicle signaling pathway39, AMPK/SIRT1/Nrf2/HO-1/NF-kB inflammatory vesicle signaling pathway40,41 and the FoxO3a/ARC/caspase-1 signaling pathway7 ; Yang F et al42 demonstrated that metformin could inhibit NLRP3 inflammatory vesicles via the AMPK/mTOR/autophagy pathway, and also that metformin could block the expression of GSDMD-N, thereby slowing down the damage caused by pyroptosis in DCM. Recent study43 found that hydrogen inhalation reduces the scorched death inflammatory response and ameliorates fibrosis by inhibiting the AMPK/mTOR/NLRP3 signaling pathway and inhibits the TGF-131/Smad signaling pathway, and that hydrogen in combination with metformin exhibits more effective cardioprotection in the treatment of DCM. In recent years, an increasing number of studies have shown that microRNAs (miRNAs), long-stranded non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) may be biomarkers or potential therapeutic targets for DCM. LncRNA Kcnq1ot1 is involved in many cardiovascular diseases, and studies have demonstrated that silencing LncRNA Kcnq1ot1 inhibits miR -214-3p/caspase-1 pathway alleviates cardiomyocyte scorching in DCM mouse models and improves cardiac function and fibrosis44 . In addition, caspase-1-related circular RNA (CACR) was increased in both high-glucose-treated cardiomyocytes and serum from diabetic patients, and CACR, as a competing endogenous RNA, promoted caspase-1 expression by targeting miR-214-3p, thereby inducing cardiomyocyte scorching, and thus CACR may be a new therapeutic target for DCM45 .ELAV-like protein 1 (ELAVL1) maybe plays a critical role in the progression of DCM, Experimental study46 have found that inhibition of miRNA-9 upregulates ELAVL1 expression and activates caspase-1. Alternatively, treatment with miRNA-9 mimics attenuates hyperglycemia-induced ELAVL1 and inhibits cardiomyocyte pyroptosis.Recent study47 found that bone morphogenetic protein-2 (BMP-2) was negatively correlated with atrial natriuretic peptide (ANP) and endogenous peptide brain natriuretic peptide (BNP) in serum of patients with type 2 diabetes combined with chronic heart failure, while in vitro experiments demonstrated that BMP-2 could protect myocardium byInhibition of NLRP3 inflammatory vesicles activation and scorching to protect cardiomyocytes, suggesting that BMP-2 may be a new target for the treatment of DCM. Some scholars further investigated48 found a novel signaling target in DCM, Nek7/GBP5 pathway activates NLRP3 inflammatory vesicles and disrupts cardiac structure and neovascularization, while BMP-7 inhibits Nek7/GBP5 pathway activation significantly reduces NLRP3 inflammatory vesicle formation, inflammatory cytokines and inflammatory cell infiltration, improving cardiac remodeling and function in DCM. However, the above signaling pathways have been less studied in DCM, and their specific mechanisms need to be further explored. With further understanding of the mechanisms of cardiomyocyte scorching, more new studies are expected to create new therapeutic approaches for patients with DCM.
Diabetic Nephropathy and Pyroptosis
DN is a chronic disease that affects approximately 40% of patients with diabetes49 It is characterized by thickening of the tubular basement membrane and glomerular membrane, proliferation of thylakoid cells, accumulation of extracellular matrix and progressive thylakoid hypertrophy50 The most critical pathological changes are tubulointerstitial inflammation and fibrosis, which are major risk factors for chronic renal failure and end-stage renal disease.51 . Hyperglycemia induces persistent chronic inflammation and plays a key role in the development and progression of DN52 ; inflammation is involved in the pathogenesis of DN through multiple pro-inflammatory cytokines, including ex vivo monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), IL-6, IL-8, and IL-1β53,54 . Persistent hyperglycemia promoting excessive production of ROS is a key factor in NLRP3 inflammatory vesicle activation. The role of NLRP3 inflammatory vesicle activation-induced pyroptosis in DN has now attracted widespread attention. Researchers have observed expression of NLRP3 and caspase-1 in podocytes and endothelial cells of kidneys from patients with diabetic nephropathy and mice55 , and that podocytes are important targets of damage in the early stages of DN. Similarly, it was shown that podocytes stimulated by high homocysteine anemia in56 or in high-fat diet-induced mouse podocytes, the formation of NLRP3 inflammatory vesicles was closely associated with the development of glomerular injury. In addition, in the stz-treated DN rat model, pyroptosis-related proteins and proinflammatory cytokines were increased, and the percentage of scorched cells was increased57 , providing further evidence for the involvement of pyroptosis in DN pathogenesis. In addition to NLRP3 inflammatory vesicle activation associated with DN pathogenesis, Wang et al.58 reported that NLRC4 inflammatory vesicles activated and secreted inflammatory cytokines under high glucose conditions, leading to scorched death of renal tubular epithelial cells. In addition, non-classical pyroptosis pathways have been shown to be involved in podocyte injury in DN mice.Cheng et al. found that high glucose treatment significantly promoted caspase-11 expression, GSDMD cleavage, and IL-1β release, whereas knockdown of caspase-11 or GSDMD attenuated these changes59 . One study60 found that NLRP3 inflammatory vesicles may play a role in high-glucose-induced glomerular thylakoid cell activation and inflammation, and that naringin alleviates glomerular thylakoid cell injury by inhibiting NLRP3/caspase-1/ IL-1β signaling pathway-mediated expression of inflammatory factors as a potential new therapy for DN. Sodium-glucose cotransport protein 2 (SGLT2) inhibitors and dipeptidyl peptidase 4 inhibitors (DPP4I) are used to treat type 2 diabetes, and recent studies have reported61 The combination of SGLT2 inhibitor and DPP4 inhibitor slows the progression of DN by inhibiting NLRP3 inflammatory vesicle activation. lncRNAs, miRNAs and circRNAs regulate the role and potential mechanisms of pyroptosis in DN development. lncRNA-MALAT1 and miR-23c have pro-scorching and anti-scorching properties in DN, respectively. The expression of MALAT1 was significantly increased and the expression of miR-23c was significantly decreased in diabetic rats and high glucose exposed HK2 cells. down-regulation of MALAT1 or up-regulation of miR-23c expression inhibited HK-2 pyroptosis. further studies revealed that miR-23c, as a target of MALAT1, directly inhibited the expression of ELAVL1 In turn, it decreased the expression of its downstream protein NLRP3, suggesting that MALAT1 is regulating the action of miR-23c on its target gene ELAVL1 to regulate renal tubular epithelial pyroptosis. It provides a new direction for the elucidation of DN pathogenesis and treatment.62,63 . Ding et al.64 found that miR-21-5p in macrophage-derived extracellular vesicles (EVs) affected cell scorch-mediated podocyte injury by increasing ROS production and activating NLRP3 inflammatory vesicles. In addition, circ_WBSCR17 was highly expressed in diabetic mice and high-glucose-treated renal tubular epithelial cells and HK-2 cells, and further studies revealed that circ_WBSCR17 exacerbated the inflammatory response and fibrosis in renal tissues by targeting and regulating the miR-185-5p/SOX6 axis65 . Cellular scorch plays an important role in renal cell injury and DN pathogenesis, including caspase-1-mediated classical cellular scorch and caspase-4/5/11-mediated non-classical cellular scorch. the NLRP3/caspase-1/GSDMD signaling axis is a key mechanism of cellular scorch in DN progression. Most current studies have been conducted in animal models. Therefore, further exploration of the detailed mechanisms and potential role of pyroptosis in renal tissue biopsies of DN patients is needed.
Diabetic Retinopathy and Pyroptosis
DR is a common chronic microvascular complication of diabetes, the pathogenesis of which remains unclear and may be associated with persistent inflammatory damage to the retinal neurovascular unit (NVU) due to a hyperglycemic state, and the chronic inflammatory response induced by pyroptosis may be involved in the pathological changes of the NVU66 . The retinal NVU is composed of retinal neurons, glial cells, vascular endothelial cells, and pericytes67 . Previous studies68 suggest that retinal neuronal degeneration or death is present in the early stages of DR and may precede microvascular lesions. It has been found that retinal ganglion pyroptosis induced by the caspase-8/HIF-1α/NLRP12/NLRP3/NLRC4 pathway under ischemic-hypoxic conditions may be a cause of retinal neuronal degeneration69,70 , and in addition, previous studies have reported71 , immunohistochemical results showed that NLRP3, ASC, and caspase-1 were specifically localized to the ganglion cell layer of the diabetic rat retina. Müller cells and astrocytes of the retinal NVU are involved in retinal structural support and maintenance of retinal homeostasis. Increased caspase-1 activity in Müller cells under high glucose conditions, increased IL-1β production, and subsequent induction of cellular scorchogenesis72 . Inhibition of the caspase-1/IL-1β pathway, on the other hand, controls the scorch death of Müller cells73 . The above evidence suggests that pyroptosis may be an important factor in Müller cell death under high glucose conditions. In addition, Müller pyroptosis leads to neuronal dysfunction and the release of pro-inflammatory factors such as IL-1β leading to endothelial cell death, breaking the integrity of the BRB and inducing cell-free capillary formation, which is one of the major pathological changes in DR74,75 . Microglia are retina-specific intrinsic immune cells that monitor the retinal microenvironment and remove metabolic waste products. Retinal ischemia-reperfusion injury is closely associated with DR progression76 , and studies have shown that77 retinal ischemia-reperfusion injury leads to the occurrence of retinal microglia focal death associated with lncRNA H19. Therefore, microglia scorch death may also be involved in the development of DR. Furthermore, recent studies78 showed that high sugar upregulated the protein expression of NLPR3, caspase-1, GSDMD, and IL-1β in retinal microglia, suggesting that high sugar induces retinal microglia scorch death through the NLPR3 inflammatory vesicle signaling pathway. Meanwhile, several studies have found that scorch death of retinal vascular endothelial cells and pericytes is involved in the pathological changes of DR. Gan J et al.79 found thathigh glucose conditions by NLPR3/caspase-1/NLPR3/caspase-1/GSDMD pathway under Scorch death mediated releases large amounts of pro-inflammatory factors leading to peripapillary retinal cell loss . the P2X7/NLRP3 pathway amplifies the inflammatory response through an ATP feedback loop under hyperglycemic and inflammation-inducing conditions, promoting inflammatory responses, pyroptosis, and apoptosis in vascular endothelial cells80 . And recent studies81 found that high glucose induces P2X7R activation of the NLPR3 inflammatory vesicle pathway leading to scorching of human retinal microvascular endothelial cells (HRMECs), whereas relaxin-3 inhibits the activation of P2X7R and NLRP3 inflammatory vesicles and delays the progression of DR. Prostaglandin E (an inflammatory mediator) induces HRMECs focal death through upregulation of NLRP3 inflammatory vesicles and inflammatory chemokine expression to promote the development of DR82 . Recent studies83 showed that downregulation of lipid transport protein 2 inhibited high glucose-induced caspase-1-mediated pyroptosis in HRMECs and slowed the progression of DR. In recent years, studies on lncRNA s, miRNAs and circRNAs in pyroptosis have provided new targets and ideas for DR prevention and treatment. circFAT1 enhances retinal pigment epithelial cell autophagy and inhibits its pyroptosis by mediating the expression of the m6A reader protein YTHDF284 . Knockdown of CircZNF532 protects retinal pigment epithelial cells from high-glucose-induced apoptosis and scorching by regulating the miR-20b-5p/STAT3 axis85 . miR-192 has been shown to be involved in DR progression by a specific mechanism that inhibits high-glucose-induced scorch death in RPE cells through regulation of the FTO/NLRP3 signaling pathway86 MiR-200c-3p is highly expressed in high-glucose-induced HRMECs, and knockdown of MiR-200c-3p attenuates high-glucose-induced scorch death in HRMECs87 , miR-200c-3p may be a potential therapeutic target for DR prevention and treatment. lncRNA myocardial infarction-associated transcript ( MIAT ) is thought to be a key regulator of DR microvascular dysfunction, and recent studies88 found that MIAT promotes caspase-1-dependent pericyte scorching by antagonizing the inhibitory effect of miR-342-3p on its target CASP1, and thus the MIAT/ miR-342-3p /CASP1 pathway may provide a new direction for the mechanism of pericyte loss and the treatment of DR.
Diabetic Peripheral Neuropathy and Pyroptosis
Diabetic peripheral neuropathy (DPN) is caused by hyperglycemia, which causes oxidative stress and inflammatory responses that damage nerve tissue. Previous studies89 demonstrated that Chevron's cells have antioxidant and anti-inflammatory neuroprotective effects, and when it becomes dysfunctional it promotes the progression of DPN, Cheng et al.89 found that strychnine prevented the scorching of RSC96 cells by inhibiting ROS production and NLRP3 inflammatory vesicle activation, suggesting that strychnine inhibited the scorching inflammatory response by antioxidant to delay the progression of DPN.
Diabetic Periodontal Disease and Pyroptosis
Diabetes can also cause periodontalhomeostasis imbalance and cause dental Periodontal disease susceptibility to the disease -Diabetic periodontal disease(DP)90 . Recent studies91 showed that high glucose activates pyroptosis via the caspase-1/GSDMD/IL-1β pathway and inhibits the proliferation and differentiation of alveolar bone osteoblasts.Zhou et al.92 found alveolar bone destruction in a mouse model of diabetic periodontitis and increased expression of GSDMD-positive cells and NLRP3 inflammatory vesicles in gingival tissue, which was partially reversed by metformin, suggesting that NLRP3-mediated scorch death has an important role in diabetic periodontitis and that gingival tissue pyroptosis may be a major cause of the inflammatory response, and that metformin treatment may improve local inflammation.2021 A study93 found that hyperglycemia induces macrophage scorch death and leads to impaired cell function and gingival destruction may be involved in the pathogenesis of diabetic periodontal disease, and that GSDMD activation and pyroptosis in the hyperglycemic state is mediated by NLRC4 inflammatory vesicles, then NLRC4 phosphorylation may be a potential therapeutic target to inhibit this process; a contemporaneous study94 demonstrated that metformin also delayed the damage to periodontal tissue from hyperglycemia-induced macrophage scorching inflammatory response.
Diabetic Keratopathy and Pyroptosis
Diabetic keratopathy (DK) is also one of the common chronic ocular complications of diabetes mellitus and is often associated with pathological changes such as delayed corneal wound healing, reduced corneal subepithelial nerve density, corneal endothelial damage, and impaired corneal perception95 More seriously, uncontrolled delayed corneal wound healing may increase susceptibility to bacterial keratitis, corneal ulceration, and even perforation96 . Under physiological conditions, NLRP3 inflammatory vesicles are necessary for corneal injury repair and nerve regeneration, however, in the case of diabetes, continued activation of NLRP3 inflammatory vesicles leads to delayed corneal wound healing and impaired nerve regeneration. Recent studies97 suggest that the pathogenesis of DK may be related to the production of accumulated AGEs by ROS promoting excessive activation of NLRP3 inflammatory vesicles. Significantly accelerating diabetic corneal epithelial wound healing and nerve regeneration by genetic and pharmacological blockade of the AGEs/ROS/NLRP3 inflammatory vesicle axis suggests that NLRP3 inflammatory vesicles are expected to be potential targets for DK treatment97 . Diabetic corneal endotheliopathy is a refractory ocular complication characterized by corneal edema and endothelial loss of compensation, which is a serious threat to vision. Long-stranded non-coding RNA KCNQ1OT1 is associated with the pathophysiological mechanisms of various complications of diabetes98,99 , recent studies confirm that the KCNQ1OT1/miR-214/caspase-1 signaling pathway may be a novel mechanism for the progression of diabetic corneal endothelial lesions and that KCNQ1OT1 has the potential to be a new therapeutic target100 .
Conclude
Only English-language literature from the WOS core database was included in this study, which may have led to a degree of bias in the search and inclusion of the study results. In addition, although the search strategy used in this study was able to cover the research articles in this field to a great extent, there is still a possibility that the search was not comprehensive. However, by systematically analyzing the literature on diabetes and pyroptosis in the Web of Science database, this paper presents a multidimensional view of the research results in this field during 2011-2022. The main features of the research in this field and the current new progress and main problems of the research in this field are pointed out, which can provide new ideas and references for the future research layout and research decisions of the research scholars in this field.
Inflammatory pyroptosis is a double-edged sword, and the challenge is whether the key proteins involved in pyroptosis can be used as a clinical biomarker to detect the progression of diabetes and its various chronic inflammatory complications, and how to safely regulate pyroptosis for the purpose of early prevention and treatment of diabetes and its complications, which require further research to explore the exact mechanisms of pyroptosis in the progression of diabetes and its complications. Taken together, research aimed at addressing these issues and innovative technologies related to them will provide new avenues and directions for the prevention and treatment of diabetes and its complications in the future.
Author Contributions
XL designed the study and drafted the manuscript as the first author. XS and XL carried out the literature search. XL contributed to data extraction and quality assessmen. XX and MX supervised the study and as the corresponding author. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China(NO:81473735) and Discipline innovation Team of Chengdu University of Traditional Chinese Medicine -- Research on the prevention and treatment of fundus diseases with traditional Chinese Medicine(XKTD2022005). The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the report.
Data Availability Statement
The original contributions presented in the study are included in the article or supplementary material. Further inquiries can be directed to the first author.
Acknowledgments
We would like to acknowledge all the authors of the research articles used for the analysis.
References
- De Candia, P.; Prattichizzo, F.; Garavelli, S.; De Rosa, V.; Galgani, M.; Di Rella, F.; Spagnuolo, M.I.; Colamatteo, A.; Fusco, C.; Micillo, T.; et al. Type 2 Diabetes: How Much of an Autoimmune Disease? Front. Endocrinol. 2019, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, B.E.; Vogel, T.P.; Bouchier-Hayes, L. Inflammatory caspase regulation: maintaining balance between inflammation and cell death in health and disease. FEBS J. 2019, 286, 2628–2644. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K. Inflammasome-associated cell death: Pyroptosis, apoptosis, and physiological implications. Microbiol. Immunol. 2020, 64, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Verdonck, S.; Nemegeer, J.; Vandenabeele, P.; Maelfait, J. Viral manipulation of host cell necroptosis and pyroptosis. Trends Microbiol. 2022, 30, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, M.; Główka, A.K.; Karaźniewicz-Łada, M. Trend research of vitamin D receptor: Bibliometric analysis. Heal. Informatics J. 2021, 27, 14604582211043158. [Google Scholar] [CrossRef]
- Schmid-Burgk, J.L.; Chauhan, D.; Schmidt, T.; Ebert, T.S.; Reinhardt, J.; Endl, E.; Hornung, V. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, N.; Zhang, Q.; Li, J.; Chen, X.; Liu, X.; Hu, Y.; Qin, W.; Shen, N.; Xu, C. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014, 5, e1479. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Lei, S.; Zhao, B.; Wu, Y.; Su, W.; Liu, M.; Meng, Q.; Zhou, B.; Leng, Y.; Xia, Z.-Y. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxidative Med. Cell. Longev. 2017, 2017, 9743280. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, M.; Zhang, Y.; An, F. NLRP3 Gene Silencing Ameliorates Diabetic Cardiomyopathy in a Type 2 Diabetes Rat Model. PLOS ONE 2014, 9, e104771. [Google Scholar] [CrossRef]
- Li, X.; Zeng, L.; Cao, C.; Lu, C.; Lian, W.; Han, J.; Zhang, X.; Zhang, J.; Tang, T.; Li, M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp. Cell Res. 2017, 350, 327–335. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qin, Y.; Wang, Y.; Meng, S.; Xian, H.; Che, H.; Lv, J.; Li, Y.; Yu, Y.; Bai, Y.; et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int. J. Biol. Sci. 2019, 15, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qin, Y.; Lv, J.; Wang, Y.; Che, H.; Chen, X.; Jiang, Y.; Li, A.; Sun, X.; Yue, E.; et al. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; He, Y.; Ming, H.; Lei, S.; Leng, Y.; Xia, Z.-Y. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J. Diabetes Res. 2019, 2019, 8151836. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Wang, Y.; Li, A.; Lv, J.; Sun, X.; Che, H.; Han, T.; Meng, S.; Bai, Y.; et al. LncRNA KCNQ1OT1 Mediates Pyroptosis in Diabetic Cardiomyopathy. Cell. Physiol. Biochem. 2018, 50, 1230–1244. [Google Scholar] [CrossRef]
- Ali, M.F.; Dasari, H.; Van Keulen, V.P.; Carmona, E.M. Canonical Stimulation of the NLRP3 Inflammasome by Fungal Antigens Links Innate and Adaptive B-Lymphocyte Responses by Modulating IL-1β and IgM Production. Front. Immunol. 2017, 8, 1504. [Google Scholar] [CrossRef] [PubMed]
- He, W.-T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-J.; Kang, Y.; Xue, Y.; Liang, X.; García, M.P.G.; Rodgers, D.; Kagel, D.R.; Du, M. Red raspberries suppress NLRP3 inflammasome and attenuate metabolic abnormalities in diet-induced obese mice. J. Nutr. Biochem. 2017, 53, 96–103. [Google Scholar] [CrossRef]
- Carlos, D.; Costa, F.R.C.; Pereira, C.A.; Rocha, F.A.; Yaochite, J.N.U.; Oliveira, G.G.; Carneiro, F.S.; Tostes, R.C.; Ramos, S.G.; Zamboni, D.S.; et al. Mitochondrial DNA Activates the NLRP3 Inflammasome and Predisposes to Type 1 Diabetes in Murine Model. Front. Immunol. 2017, 8, 164. [Google Scholar] [CrossRef]
- Iannantuoni, F.; Diaz-Morales, N.; Escribano-Lopez, I.; Sola, E.; Roldan-Torres, I.; Apostolova, N.; Bañuls, C.; Rovira-Llopis, S.; Rocha, M.; Victor, V.M. Does Glycemic Control Modulate the Impairment of NLRP3 Inflammasome Activation in Type 2 Diabetes? Antioxidants Redox Signal. 2019, 30, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hao, H.; Han, Q.; Song, X.; Liu, J.; Dong, L.; Han, W.; Mu, Y. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res. Ther. 2017, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, X.; Yang, C.; Nie, W.; Zhang, J.; Li, H.; Rong, P.; Yi, S.; Wang, W. Hypoxia potentiates LPS-induced inflammatory response and increases cell death by promoting NLRP3 inflammasome activation in pancreatic β cells. Biochem. Biophys. Res. Commun. 2017, 495, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Li, X.; Li, Y. The role of TRIM family proteins in autophagy, pyroptosis, and diabetes mellitus. Cell Biol. Int. 2021, 45, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.M.; Shinagawa, T.; Ishii, S. Trim27-deficient mice are susceptible to streptozotocin-induced diabetes. FEBS Open Bio, 2013; 4, 60–64. [Google Scholar]
- Cohen, S.; Lee, D.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. Trim32 reduces PI3K-Akt-FoxO signaling in muscle atrophy by promoting plakoglobin-PI3K dissociation. J. Cell Biol. 2014, 204, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Montori-Grau, M.; Pedreira-Casahuga, R.; Boyer-Diaz, Z.; Lassot, I.; Garcia-Martinez, C.; Orozco, A.; Cebria, J.; Osorio-Conles, O.; Chacon, M.R.; Vendrell, J.; et al. GNIP1 E3 ubiquitin ligase is a novel player in regulating glycogen metabolism in skeletal muscle. Metabolism 2018, 83, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Peng, W.; Zhang, Y.; Lv, F.; Wu, H.-K.; Guo, J.; Cao, Y.; Pi, Y.; Zhang, X.; Jin, L.; et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 2013, 494, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, D.; Shen, Y.; Zheng, X.; Xu, G. Altered DNA methylation of TRIM13 in diabetic nephropathy suppresses mesangial collagen synthesis by promoting ubiquitination of CHOP. EBioMedicine 2020, 51, 102582. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2003, 18, 39–51. [Google Scholar] [CrossRef]
- Prandi, F.R.; Evangelista, I.; Sergi, D.; Palazzuoli, A.; Romeo, F. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants. Hear. Fail. Rev. 2022, 28, 597–606. [Google Scholar] [CrossRef]
- Prabhu, S.; Frangogiannis, N.J. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, L.; Yang, K.; Fu, Y.; Liu, Y.; Chi, J.; Zhang, X.; Hong, S.; Ma, X.; Yin, X. H3 Relaxin Protects Against Myocardial Injury in Experimental Diabetic Cardiomyopathy by Inhibiting Myocardial Apoptosis, Fibrosis and Inflammation. Cell. Physiol. Biochem. 2017, 43, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.; Zong, B.; Yuan, H.; Wang, Z.; Wei, Y.; Wang, X.; Liu, G.; Zhang, J.; Li, S.; et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J. Cell. Mol. Med. 2018, 22, 4437–4448. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, L.; Wang, Q.; Shao, X.; Luo, Q.; Liu, S.; Li, Y.; Wang, D.; Zhang, Y.; Diao, H.; et al. The Chinese herbal medicine Fufang Zhenzhu Tiaozhi protects against diabetic cardiomyopathy by alleviating cardiac lipotoxicity-induced oxidative stress and NLRP3-dependent inflammasome activation. Biomed. Pharmacother. 2022, 148, 112709. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Kambis, T.N.; Kar, S.; Park, S.Y.; Mishra, P.K. MMP9 mediates acute hyperglycemia-induced human cardiac stem cell death by upregulating apoptosis and pyroptosis in vitro. Cell Death Dis. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yuan, S.; Luan, X.; Feng, J.; Deng, L.; Zuo, Y.; Li, J. Pyroptosis-Related Inflammasome Pathway: A New Therapeutic Target for Diabetic Cardiomyopathy. Front. Pharmacol. 2022, 13, 842313. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Wang, X.; Li, P.; Zhang, S.; Wu, T.; Yan, Y.; Zhan, Y.; Ren, Y.; Rong, X.; et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem. -Biol. Interact. 2019, 310, 108754. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Wang, Y.; Meng, S.; Xian, H.; Che, H.; Lv, J.; Li, Y.; Yu, Y.; Bai, Y.; et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int. J. Biol. Sci. 2019, 15, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Nie, C.; Pan, S.; Wang, B.; Hong, X.; Xi, S.; Bai, J.; Yu, M.; Liu, J.; Yang, W. Co-administration of hydrogen and metformin exerts cardioprotective effects by inhibiting pyroptosis and fibrosis in diabetic cardiomyopathy. Free. Radic. Biol. Med. 2022, 183, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qin, Y.; Wang, Y.; Li, A.; Lv, J.; Sun, X.; Che, H.; Han, T.; Meng, S.; Bai, Y.; et al. LncRNA KCNQ1OT1 Mediates Pyroptosis in Diabetic Cardiomyopathy. Cell. Physiol. Biochem. 2018, 50, 1230–1244. [Google Scholar] [CrossRef]
- Yang, F.; Li, A.; Qin, Y.; Che, H.; Wang, Y.; Lv, J.; Li, Y.; Li, H.; Yue, E.; Ding, X.; et al. A Novel Circular RNA Mediates Pyroptosis of Diabetic Cardiomyopathy by Functioning as a Competing Endogenous RNA. Mol. Ther. - Nucleic Acids 2019, 17, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Jeyabal, P.; Thandavarayan, R.A.; Joladarashi, D.; Babu, S.S.; Krishnamurthy, S.; Bhimaraj, A.; Youker, K.A.; Kishore, R.; Krishnamurthy, P. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem. Biophys. Res. Commun. 2016, 471, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Yu, R.-Q.; Wu, F.-Z.; Qiao, L.; Wu, X.-R.; Fu, Y.-J.; Liang, Y.-F.; Pang, Y.; Xie, C.-Y. BMP-2 alleviates heart failure with type 2 diabetes mellitus and doxorubicin-induced AC16 cell injury by inhibiting NLRP3 inflammasome-mediated pyroptosis. Exp. Ther. Med. 2021, 22, 897. [Google Scholar] [CrossRef]
- Elmadbouh, I.; Singla, D.K. BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy. Cells 2021, 10, 2640. [Google Scholar] [CrossRef] [PubMed]
- Dagar, N.; Das, P.; Bisht, P.; Taraphdar, A.K.; Velayutham, R.; Arumugam, S. Diabetic nephropathy: A twisted thread to unravel. Life Sci. 2021, 278, 119635. [Google Scholar] [CrossRef]
- Ni, W.; Tang, L.; Wei, W. Research progress in signalling pathway in diabetic nephropathy. Diabetes/Metabolism Res. Rev. 2015, 31, 221–233. [Google Scholar] [CrossRef]
- Liu, B.-C.; Tang, T.-T.; Lv, L.-L.; Lan, H.-Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Warren, A.M.; Knudsen, S.T.; Cooper, M.E. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin. Ther. Targets 2019, 23, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Gao, G. High glucose induces rat mesangial cells proliferation and MCP-1 expression via ROS-mediated activation of NF-κB pathway, which is inhibited by eleutheroside E. J. Recept. Signal Transduct. 2016, 36, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, P.; Bi, Y.; Chen, J.; Xiao, Z.; Zhang, X.; Wang, Z. Danshen injection ameliorates STZ-induced diabetic nephropathy in association with suppression of oxidative stress, pro-inflammatory factors and fibrosis. Int. Immunopharmacol. 2016, 38, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019, 96, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Boini, K.M.; Xia, M.; Abais, J.M.; Li, X.; Liu, Q.; Li, P.-L.; H, H.; Y, W.; X, L.; et al. Activation of Nod-Like Receptor Protein 3 Inflammasomes Turns on Podocyte Injury and Glomerular Sclerosis in Hyperhomocysteinemia. Hypertension 2012, 60, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.-F.; Huang, H.-W.; Huang, C.; Hu, L.-L.; Xu, W.-W. Long Non-Coding RNA NEAT1 Regulates Pyroptosis in Diabetic Nephropathy via Mediating the miR-34c/NLRP3 Axis. Kidney Blood Press. Res. 2020, 45, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gou, R.; Yu, L.; Wang, L.; Yang, Z.; Guo, Y.; Tang, L. Activation of the NLRC4 inflammasome in renal tubular epithelial cell injury in diabetic nephropathy. Exp. Ther. Med. 2021, 22, 814. [Google Scholar] [CrossRef]
- Cheng, Q.; Pan, J.; Zhou, Z.L.; Yin, F.; Xie, H.Y.; Chen, P.P.; Li, J.Y.; Zheng, P.Q.; Zhou, L.; Zhang, W.; et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol. Sin. 2021, 42, 954–963. [Google Scholar] [CrossRef]
- Chen, F.; Wei, G.; Xu, J.; Ma, X.; Wang, Q. Naringin ameliorates the high glucose-induced rat mesangial cell inflammatory reaction by modulating the NLRP3 Inflammasome. BMC Complement. Altern. Med. 2018, 18, 192. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Bajaj, M.; Yang, H.C.; Ye, Y. Combined SGLT2 and DPP4 Inhibition Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Nephropathy in Mice with Type 2 Diabetes. Cardiovasc. Drugs Ther. 2018, 32, 135–145. [Google Scholar] [CrossRef]
- Li, X.; Zeng, L.; Cao, C.; Lu, C.; Lian, W.; Han, J.; Zhang, X.; Zhang, J.; Tang, T.; Li, M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp. Cell Res. 2017, 350, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhuo, H.; Ye, M.; Huang, G.; Fan, M.; Huang, X. LncRNA MALAT1 promoted high glucose-induced pyroptosis of renal tubular epithelial cell by sponging miR-30c targeting for NLRP3. Kaohsiung J. Med Sci. 2020, 36, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jing, N.; Shen, A.; Guo, F.; Song, Y.; Pan, M.; Ma, X.; Zhao, L.; Zhang, H.; Wu, L.; et al. MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J. Endocrinol. Investig. 2021, 44, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qin, Y.; Qin, S.; Zhou, X.; Zhao, W.; Zhang, D. Circ_WBSCR17 aggravates inflammatory responses and fibrosis by targeting miR-185-5p/SOX6 regulatory axis in high glucose-induced human kidney tubular cells. Life Sci. 2020, 259, 118269. [Google Scholar] [CrossRef] [PubMed]
- Xiaodong, L.; Xuejun, X. GSDMD-mediated pyroptosis in retinal vascular inflammatory diseases: a review. Int. Ophthalmol. 2022, 43, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.-P. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Dehghani, C.; Pritchard, N.; Edwards, K.; Russell, A.W.; Malik, R.A.; Efron, N. Corneal and Retinal Neuronal Degeneration in Early Stages of Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2017, 58, 6365–6373. [Google Scholar] [CrossRef]
- Thomas, C.N.; Berry, M.; Logan, A.; Blanch, R.J.; Ahmed, Z. Caspases in retinal ganglion cell death and axon regeneration. Cell Death Discov. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Chen, H.; Deng, Y.; Gan, X.; Li, Y.; Huang, W.; Lu, L.; Wei, L.; Su, L.; Luo, J.; Zou, B.; et al. NLRP12 collaborates with NLRP3 and NLRC4 to promote pyroptosis inducing ganglion cell death of acute glaucoma. Mol. Neurodegener. 2020, 15, 26. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, F.; Wang, W.; Wang, H.; Zhang, X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol. Vis. 2017, 23, 242–250. [Google Scholar]
- Trueblood, K.E.; Mohr, S.; Dubyak, G.R.; Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Purinergic regulation of high-glucose-induced caspase-1 activation in the rat retinal Müller cell line rMC-1. Am. J. Physiol. Physiol. 2011, 301, C1213–C1223. [Google Scholar] [CrossRef] [PubMed]
- Yego, D.J.F.E.C.; Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller cells and diabetic retinopathy. Vis. Res. 2017, 139, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Busik, J.V.; Mohr, S.; Grant, M.B. Hyperglycemia-Induced Reactive Oxygen Species Toxicity to Endothelial Cells Is Dependent on Paracrine Mediators. Diabetes 2008, 57, 1952–1965. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, M.J.; Cho, H.; Wu, L.; Chen, W.J.; Gong, J.; Duh, E.J. A Mouse Model of Retinal Ischemia-Reperfusion Injury Through Elevation of Intraocular Pressure. JoVE 2016, 113, e54065. [Google Scholar]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Deng, C.; Li, J.; Jiang, N.; Huang, S.; Long, E.; Zhuo, Y. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ. 2019, 27, 176–191. [Google Scholar] [CrossRef]
- Huang, L.; You, J.; Yao, Y.; Xie, M. High glucose induces pyroptosis of retinal microglia through NLPR3 inflammasome signaling. Arq. Bras. de Oftalmol. 2020, 84, 67–73. [Google Scholar] [CrossRef]
- Gan, J.; Huang, M.; Lan, G.; Liu, L.; Xu, F. High Glucose Induces the Loss of Retinal Pericytes Partly via NLRP3-Caspase-1-GSDMD-Mediated Pyroptosis. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Kong, H.; Zhao, H.; Chen, T.; Song, Y.; Cui, Y. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022, 13, 336. [Google Scholar] [CrossRef]
- Yang, K.; Liu, J.; Zhang, X.; Ren, Z.; Gao, L.; Wang, Y.; Lin, W.; Ma, X.; Hao, M.; Kuang, H. H3 Relaxin Alleviates Migration, Apoptosis and Pyroptosis Through P2X7R-Mediated Nucleotide Binding Oligomerization Domain-Like Receptor Protein 3 Inflammasome Activation in Retinopathy Induced by Hyperglycemia. Front. Pharmacol. 2020, 11, 603689. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Yao, Y. Prostaglandin E2 Activates NLRP3 Inflammasome in Endothelial Cells to Promote Diabetic Retinopathy. Horm. Metab. Res. 2018, 50, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhou, P.; Qi, Y. Down-regulation of LCN2 attenuates retinal vascular dysfunction and caspase-1-mediated pyroptosis in diabetes mellitus. Ann. Transl. Med. 2022, 10, 695–695. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qi, P.; Cui, H.; Lu, Q.; Gao, X. CircFAT1 regulates retinal pigment epithelial cell pyroptosis and autophagy via mediating m6A reader protein YTHDF2 expression in diabetic retinopathy. Exp. Eye Res. 2022, 222, 109152. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Luo, Y.; Wei, R.; Yin, J.; Qin, Z.; Lu, L.; Ma, W. CircZNF532 knockdown protects retinal pigment epithelial cells against high glucose-induced apoptosis and pyroptosis by regulating the miR-20b-5p/STAT3 axis. J. Diabetes Investig. 2021, 13, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, H.; Li, Q.; Zhao, S.; Gao, Y. MiR-192 attenuates high glucose-induced pyroptosis in retinal pigment epithelial cells via inflammasome modulation. Bioengineered 2022, 13, 10362–10372. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, S.; Chen, G.; He, S. MiR-200c-3p regulates pyroptosis by targeting SLC30A7 in diabetic retinopathy. Hum. Exp. Toxicol. 2022, 41, 9603271221099589. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ma, X.; Lin, W.; Xu, Q.; Zhou, H.; Kuang, H. Long noncoding RNA MIAT regulates primary human retinal pericyte pyroptosis by modulating miR-342–3p targeting of CASP1 in diabetic retinopathy. Exp. Eye Res. 2020, 202, 108300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Chu, L.-W.; Chen, J.-Y.; Hsieh, S.-L.; Chang, Y.-C.; Dai, Z.-K.; Wu, B.-N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Shan, Q.; Geng, G.; Shao, P. High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 471–478. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Q.; Nie, L.; Zhang, P.; Zhao, P.; Yuan, Q.; Ji, N.; Ding, Y.; Wang, Q. Metformin ameliorates the NLPP3 inflammasome mediated pyroptosis by inhibiting the expression of NEK7 in diabetic periodontitis. Arch. Oral Biol. 2020, 116, 104763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yue, Z.; Nie, L.; Zhao, Z.; Wang, Q.; Chen, J.; Wang, Q. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J. Clin. Periodontol. 2021, 48, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Zhao, P.; Yue, Z.; Zhang, P.; Ji, N.; Chen, Q.; Wang, Q. Diabetes induces macrophage dysfunction through cytoplasmic dsDNA/AIM2 associated pyroptosis. J. Leukoc. Biol. 2021, 110, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int. J. Biol. Sci. 2022, 18, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Luo, H.; Liu, B.; Li, F.; Tschöpe, C.; Fa, X. Long noncoding RNAs: A new player in the prevention and treatment of diabetic cardiomyopathy? Diabetes/Metab. Res. Rev. 2018, 34, e3056. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Lv, J.; Wang, Y.; Che, H.; Chen, X.; Jiang, Y.; Li, A.; Sun, X.; Yue, E. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Z.; Li, X.; Xu, S.; Zhou, S.; Jin, X.; Zhang, H. Long noncoding RNA KCNQ1OT1 induces pyroptosis in diabetic corneal endothelial keratopathy. Am. J. Physiol. Physiol. 2020, 318, C346–C359. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer's disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Goldwaser, E.L.; Acharya, N.K.; Sarkar, A.; Godsey, G.; Nagele, R.G. Breakdown of the Cerebrovasculature and Blood-Brain Barrier: A Mechanistic Link Between Diabetes Mellitus and Alzheimer’s Disease. J. Alzheimer's Dis. 2016, 54, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Chen, H.; Li, F.; Zhu, Y.; Yin, W.; Zhuo, Y. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J. Neuroinflammation 2015, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.S.; Zou, Q.C.; Xiong, W.J.; Cui, N.Y.; Wang, K.; Liu, H.X.; Lou, W.J.; Higazy, D.; Zhang, Y.G.; Cui, M. Brain Microvascular Endothelial Cell-Derived HMGB1 Facilitates Monocyte Adhesion and Transmigration to Promote JEV Neuroinvasion. Front. Cell. Infect. Microbiol. 2021, 11, 701820. [Google Scholar]
- Zhang, T.; Sun, L.; Wang, T.; Liu, C.; Zhang, H.; Zhang, C.; Yu, L. Gestational exposure to PM2.5 leads to cognitive dysfunction in mice offspring via promoting HMGB1-NLRP3 axis mediated hippocampal inflammation. Ecotoxicol. Environ. Saf. 2021, 223, 112617. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shi, Y.; Du, P.; Wang, J.; Han, Y.; Sun, B.; Feng, J. HMGB1/TLR4 promotes apoptosis and reduces autophagy of hippocampal neurons in diabetes combined with OSA. Life Sci. 2019, 239, 117020. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Guo, C.; Song, F.; Huo, Y.; Geng, Y.; Guo, M.; Bao, H.; Wu, X.; Fan, W. Mild hypothermia alleviates diabetes aggravated cerebral ischemic injury via activating autophagy and inhibiting pyroptosis. Brain Res. Bull. 2019, 150, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, J.; Qin, A.; Chen, Z. The protective effect of formononetin on cognitive impairment in streptozotocin (STZ)-induced diabetic mice. Biomed. Pharmacother. 2018, 106, 1250–1257. [Google Scholar] [CrossRef]
Figure 1.
Infographic of the number of publications and frequency of citations in the field of diabetes and pyroptosis over the years.
Figure 1.
Infographic of the number of publications and frequency of citations in the field of diabetes and pyroptosis over the years.
Figure 2.
Top 10 countries/regions in terms of cumulative number of publications.
Figure 2.
Top 10 countries/regions in terms of cumulative number of publications.
Figure 3.
Distribution of collaborative relationships in published literature by coun try/region.
Figure 3.
Distribution of collaborative relationships in published literature by coun try/region.
Figure 4.
Top 10 scientific institutions in the field of diabetes and pyroptosis in terms of number of publications.
Figure 4.
Top 10 scientific institutions in the field of diabetes and pyroptosis in terms of number of publications.
Figure 5.
Top 10 highly cited scientific institutions in published literature in the field of diabetes and pyroptosis.
Figure 5.
Top 10 highly cited scientific institutions in published literature in the field of diabetes and pyroptosis.
Figure 6.
Collaboration of scientific institutions publishing literature in the field of diabetes and pyroptosis.
Figure 6.
Collaboration of scientific institutions publishing literature in the field of diabetes and pyroptosis.
Figure 7.
Distribution of collaborative relationships between authors of published literature on diabetes and pyroptosis studies.
Figure 7.
Distribution of collaborative relationships between authors of published literature on diabetes and pyroptosis studies.
Figure 8.
Top 15 keywords in cumulative frequency of published literature on diabetes and pyroptosis research.
Figure 8.
Top 15 keywords in cumulative frequency of published literature on diabetes and pyroptosis research.
Figure 9.
Cluster analysis network relationship diagram of high frequency keywords in published literature on diabetes and pyroptosis research.
Figure 9.
Cluster analysis network relationship diagram of high frequency keywords in published literature on diabetes and pyroptosis research.
Figure 10.
Distribution of keyword density in published literature on diabetes and pyroptosis studies.
Figure 10.
Distribution of keyword density in published literature on diabetes and pyroptosis studies.
Figure 11.
Trends in diabetes and pyroptosis research themes, 2011-2022.
Figure 11.
Trends in diabetes and pyroptosis research themes, 2011-2022.
Figure 12.
Proportional distribution of the number of publications per year in diabetes complications and pyroptosis studies, 2014-2022 (Note:DCM:Diabetic cardiomyopathy;DN:Diabetic nephropathy;DR:Diabetic retinopathy;DACD:Diabetes-related cognitive decline;DCAN: diabetic cardiac autonomic neuropathy;DMED:diabetes mellitus-induced erectile dysfunction;DPN:Diabetic peripheral neuropathy;DP:Diabetic periodontal diseaseDK:Diabetic keratopathy).
Figure 12.
Proportional distribution of the number of publications per year in diabetes complications and pyroptosis studies, 2014-2022 (Note:DCM:Diabetic cardiomyopathy;DN:Diabetic nephropathy;DR:Diabetic retinopathy;DACD:Diabetes-related cognitive decline;DCAN: diabetic cardiac autonomic neuropathy;DMED:diabetes mellitus-induced erectile dysfunction;DPN:Diabetic peripheral neuropathy;DP:Diabetic periodontal diseaseDK:Diabetic keratopathy).
Table 1.
Table of information on the top 10 highly cited publications in the field of diabetes and pyroptosis.
Table 1.
Table of information on the top 10 highly cited publications in the field of diabetes and pyroptosis.
| first author |
annual |
national |
periodical |
Total number of citations |
Average |
| Schmid-Burgk, JL6
|
2016 |
German |
JOURNAL OF BIOLOGICAL CHEMISTRY |
255 |
36.43 |
| Li, X7
|
2014 |
sino |
CELL DEATH & DISEASE |
190 |
21.11 |
| Qiu,Z8
|
2017 |
sino |
OXIDATIVE MEDICINE AND CELLULAR LONGEVITY |
177 |
29.50 |
| Luo, BB9
|
2014 |
sino |
PLOS ONE |
166 |
18.44 |
| Li, X10
|
2017 |
sino |
EXPERIMENTAL CELL RESEARCH |
159 |
26.50 |
| Giordano, A11
|
2013 |
Italy |
JOURNAL OF LIPID RESEARCH |
154 |
15.40 |
| Yang, F12
|
2019 |
sino |
INTERNATIONAL JOURNAL OF BIOLOGICAL SCIENCES |
122 |
30.50 |
| Yang, F13
|
2018 |
sino |
CELL DEATH & DISEASE |
117 |
23.40 |
| Qiu,Z14
|
2019 |
sino |
OURNAL OF DIABETES RESEARCH |
109 |
27.25 |
| Yang, F15
|
2018 |
sino |
CELLULAR PHYSIOLOGY AND BIOCHEMISTRY |
90 |
18 |
Table 2.
Top 10 authors with high citations in published literature on diabetes and pyroptosis research.
Table 2.
Top 10 authors with high citations in published literature on diabetes and pyroptosis research.
| author name |
Total literature |
Total number of citations |
Average number of citations |
Number of operations |
Number of communications |
| Chen, X |
5 |
29 |
5.80 |
0 |
0 |
| Wang, Y Q |
4 |
29 |
7.25 |
0 |
0 |
| Che, H |
4 |
29 |
7.25 |
1 |
0 |
| Wang, L H |
4 |
29 |
7.25 |
0 |
3 |
| Yang, F |
3 |
28 |
9.33 |
3 |
0 |
| Qin, Y |
4 |
28 |
7.00 |
0 |
0 |
| Lv, J |
3 |
28 |
9.33 |
0 |
0 |
| Bai, Y L |
6 |
28 |
4.67 |
1 |
1 |
| Zhang, Y |
6 |
28 |
4.67 |
0 |
1 |
| Li, X |
4 |
23 |
5.75 |
2 |
0 |
Table 3.
Information on the top 10 journals in terms of cumulative number of publications in diabetes and pyroptosis research.
Table 3.
Information on the top 10 journals in terms of cumulative number of publications in diabetes and pyroptosis research.
| Journal Name |
Number of articles issued |
impact factor |
CAS Division |
H-index |
| CELL DEATH DISEASE |
4 |
9.685 |
first |
59 |
| BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS |
4 |
3.322 |
third |
43 |
| FRONTIERS IN PHARMACOLOGY |
4 |
5.988 |
second |
57 |
| INTERNATIONAL IMMUNOPHARMACOLOGY |
4 |
5.714 |
second |
40 |
| LIFE SCIENCES |
4 |
6.780 |
third |
51 |
| OXIDATIVE MEDICINE AND CELLULAR LONGEVITY |
4 |
7.310 |
second |
49 |
| BIOENGINEERED |
3 |
6.832 |
quadrant |
22 |
| BIOMEDICINE PHARMACOTHERAPY |
3 |
7.419 |
second |
59 |
| JOURNAL OF DIABETES INVESTIGATION |
3 |
3.681 |
third |
22 |
| JOURNAL OF DIABETES RESEARCH |
3 |
4.061 |
third |
24 |
Table 4.
Information on the top 10 highly cited journals for published literature on diabetes and pyroptosis research.
Table 4.
Information on the top 10 highly cited journals for published literature on diabetes and pyroptosis research.
| Journal Name |
Number of articles issued |
Total number of citations |
Average number of citations |
CAS Division |
| CELL DEATH DISEASE |
4 |
29 |
7.25 |
first |
| PLOS ONE |
2 |
14 |
7.00 |
third |
| OXIDATIVE MEDICINE AND CELLULAR LONGEVITY |
4 |
11 |
2.75 |
second |
| CELLULAR PHYSIOLOGY AND BIOCHEMISTRY |
1 |
8 |
8.00 |
uncatalogued |
| INTERNATIONAL JOURNAL OF BIOLOGICAL SCIENCES |
2 |
7 |
3.50 |
second |
| BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS |
4 |
6 |
1.50 |
third |
| JOURNAL OF DIABETES RESEARCH |
3 |
6 |
2.00 |
third |
| EXPERIMENTAL CELL RESEARCH |
1 |
6 |
6.00 |
third |
| JOURNAL OF LIPID RESEARCH |
2 |
5 |
2.50 |
second |
| BIOMED RESEARCH INTERNATIONAL |
1 |
3 |
3.00 |
third |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).