Submitted:
04 January 2023
Posted:
06 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
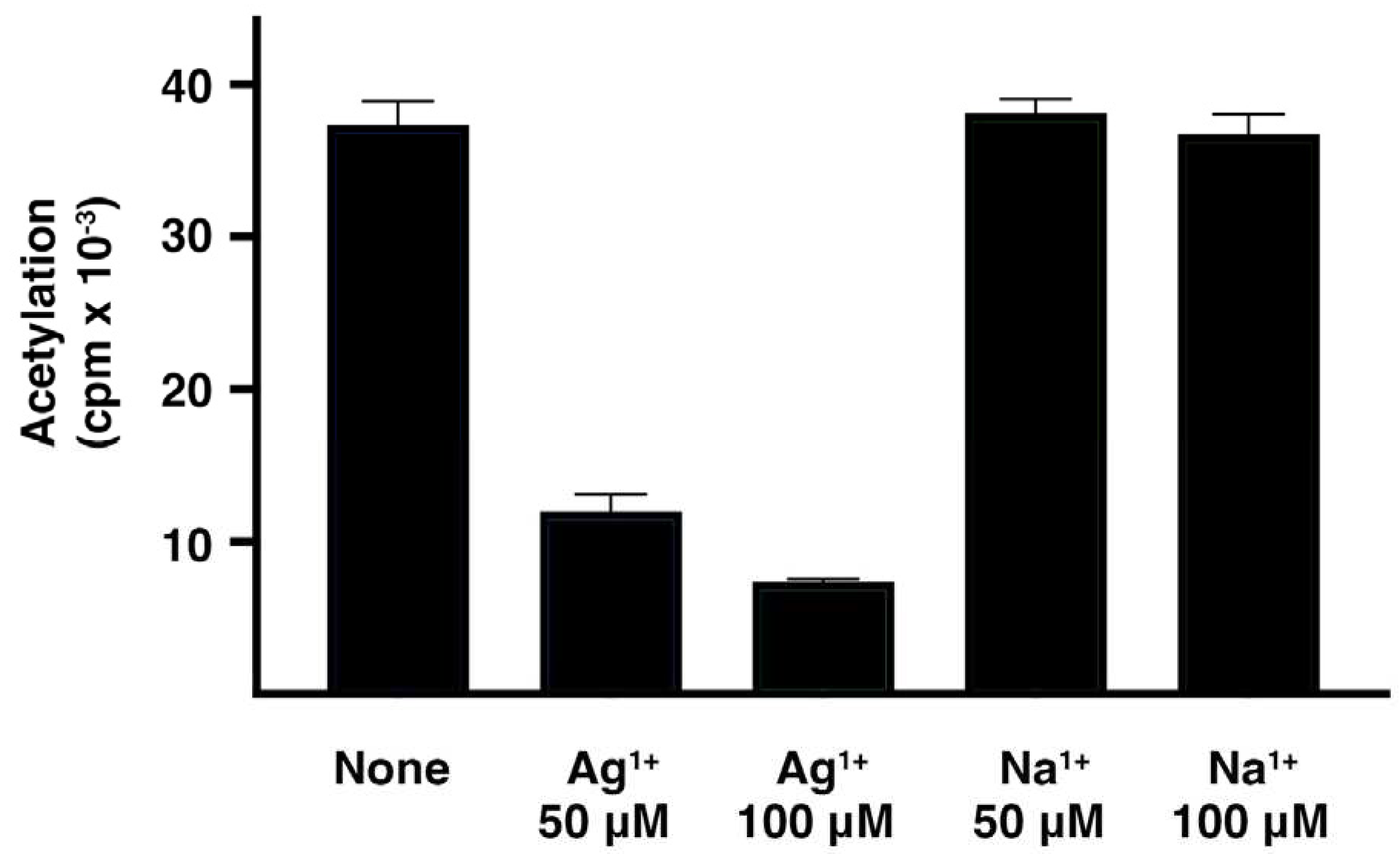
2.1. Effect of Ag1+ on AAC(2')-Ia-mediated acetylation of plazomicin
2.2. Effect of Ag1+ on AAC(2')-Ia-mediated resistance to plazomicin
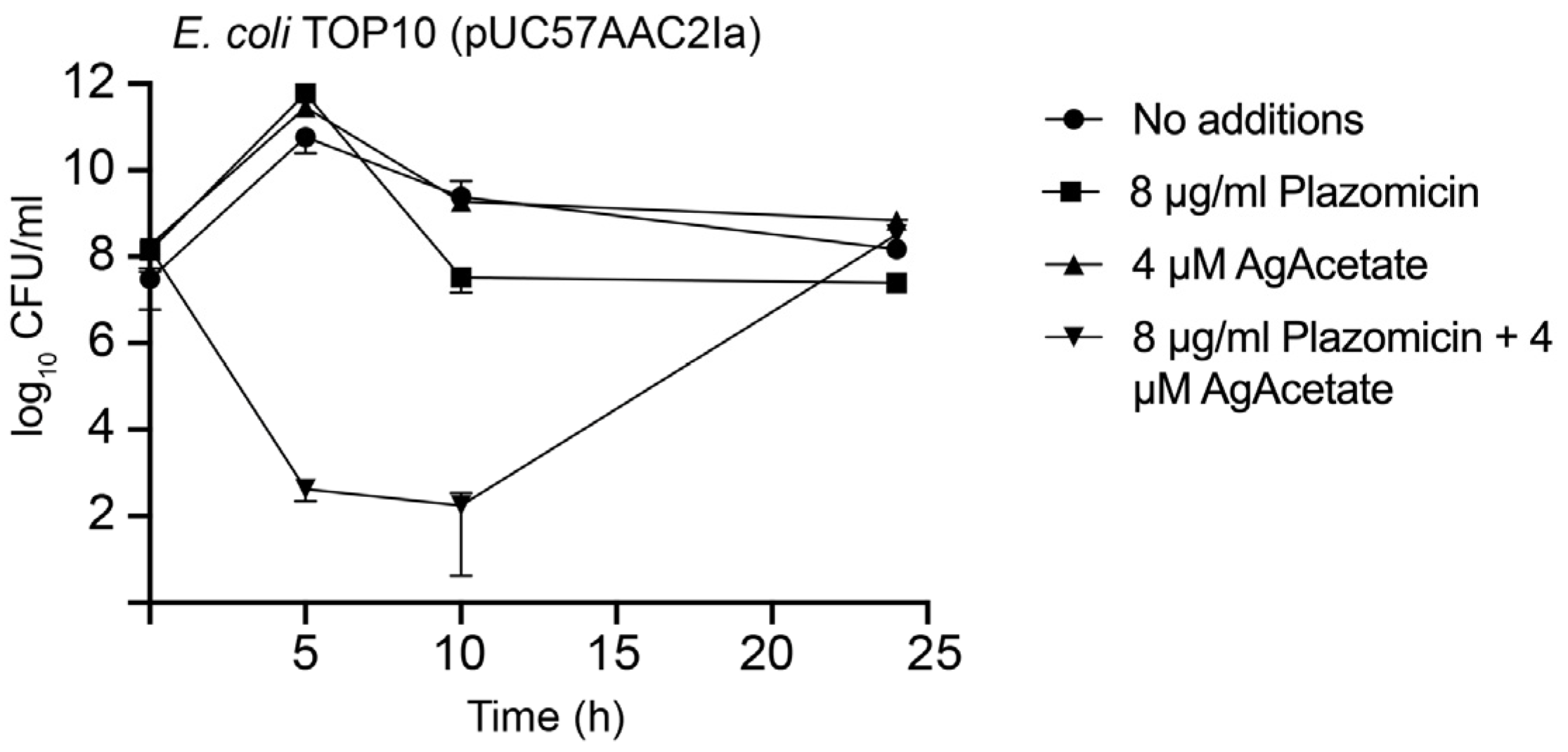
2.3. Bactericidal effect
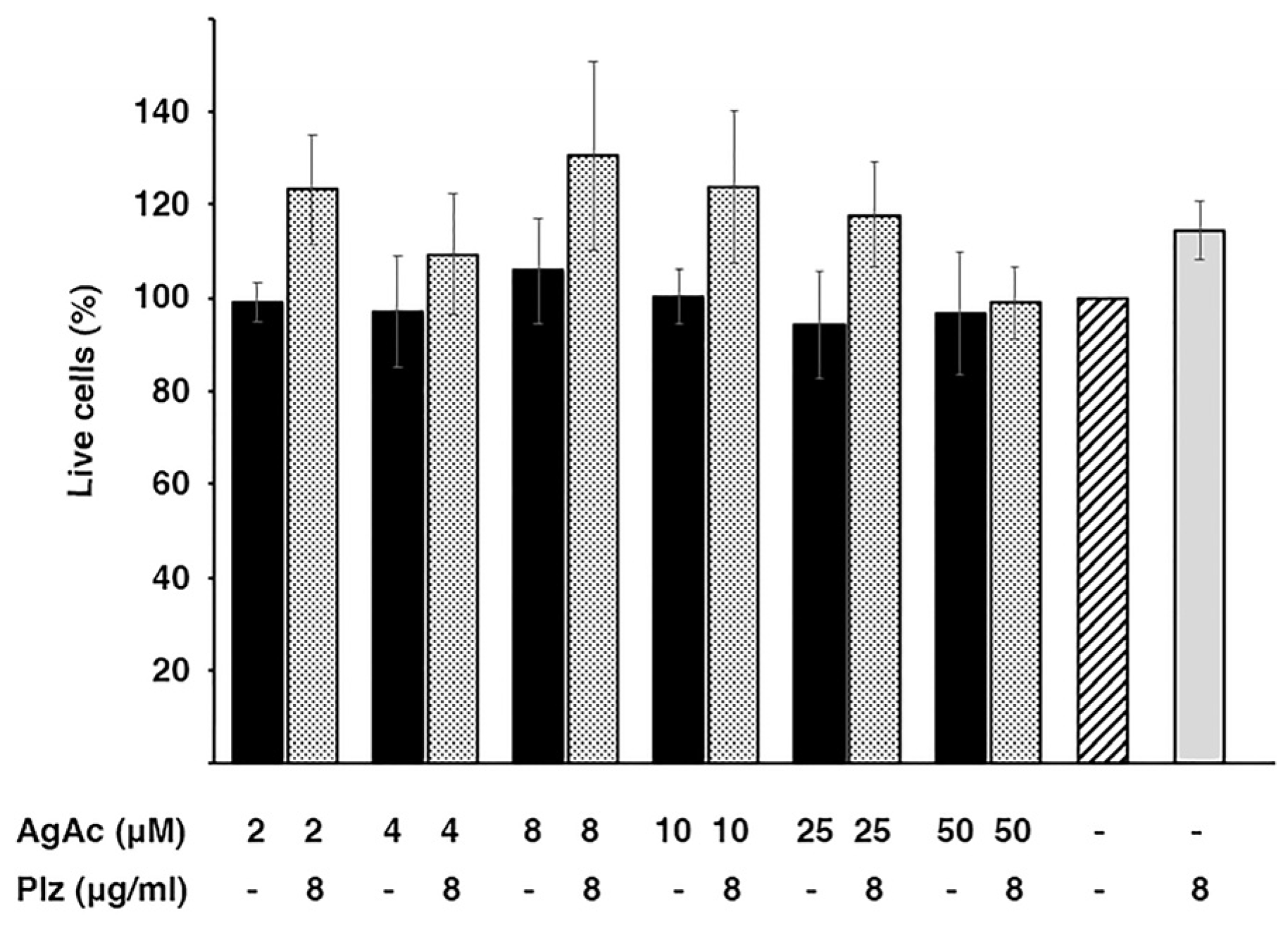
2.3. Cytotoxicity of the mix plazomicin/silver acetate
3. Discussion
4. Materials and Methods
4.1. Bacterial strains and plasmids
4.2. Bacterial growth
4.3. MIC determination
4.4. Time-kill assays
4.5. Acetyltransferase assays
4.6. Cytotoxicity assays
5. Conclusions
Author Contributions
Funding
Acknowledgments
References
- Boucher, H. W. Bad bugs, no drugs 2002-2020: progress, challenges, and call to action. Trans Am Clin Climatol Assoc 2020, 131, 65–71. [Google Scholar] [PubMed]
- Adler, A.; Friedman, N. D.; Marchaim, D. Multidrug-resistant Gram-negative bacilli: infection control implications. Infect Dis Clin North Am 2016, 30, 967–997. [Google Scholar] [CrossRef]
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J Antimicrob Chemother 2021, 76, iv23–iv37. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; El Chakhtoura, N. G.; Yasmin, M.; Bonomo, R. A. Polymyxins: to combine or not to Combine? Antibiotics (Basel) 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Machuca, I.; Gutierrez-Gutierrez, B.; Rivera-Espinar, F.; Cano, A.; Gracia-Ahufinger, I.; Guzman-Puche, J.; Marfil-Perez, E.; Perez-Nadales, E.; Caston, J. J.; Bonomo, R. A.; Carmeli, Y.; Paterson, D.; Pascual, A.; Martinez-Martinez, L.; Rodriguez-Bano, J.; Torre-Cisneros, J.; Group, R. E. I. External validation of the INCREMENT-CPE mortality score in a carbapenem-resistant Klebsiella pneumoniae bacteraemia cohort: the prognostic significance of colistin resistance. Int J Antimicrob Agents 2019, 54, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Santiago, J.; Cornejo-Juarez, P.; Silva-Sanchez, J.; Garza-Ramos, U. Polymyxin resistance in Enterobacterales: overview and epidemiology in the Americas. Int J Antimicrob Agents 2021, 58, 106426. [Google Scholar] [CrossRef] [PubMed]
- Burillo, A.; Bouza, E. Controversies over the management of infections caused by Amp-C- and ESBL-producing Enterobacterales: what questions remain for future studies? Curr Opin Infect Dis 2022, 35, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yamawaki, K. Cefiderocol: discovery, chemistry, and In vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 2019, 69, S538–S543. [Google Scholar] [CrossRef]
- Mezcord, V.; Wong, O.; Pasteran, F.; Corso, A.; Tolmasky, M. E.; Bonomo, R. A.; Ramirez, M. S. Role of beta-lactamase inhibitors on cefiderocol activity against carbapenem-resistant Acinetobacter species. Int J Antimicrob Agents 2022, 106700. [Google Scholar]
- Ramirez, M. S.; Tolmasky, M. E. Aminoglycoside modifying enzymes. Drug Resist Updat 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Labby, K. J.; Garneau-Tsodikova, S. Strategies to overcome the action of aminoglycoside-modifying enzymes for treating resistant bacterial infections. Future Med Chem 2013, 5, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M. S.; Nikolaidis, N.; Tolmasky, M. E. Rise and dissemination of aminoglycoside resistance: the aac(6')-Ib paradigm. Front Microbiol 2013, 4, 121. [Google Scholar] [CrossRef]
- Tolmasky, M. E. Strategies to prolong the useful life of existing antibiotics and help overcoming the antibiotic resistance crisis. In Frontiers in Clinical Drug Research-Anti Infectives; Atta-ur-Rhaman, Ed.; Bentham Books: Sharjah, UAE, 2017; Volume 1, pp. 1–27. [Google Scholar]
- Ramirez, M. S.; Tolmasky, M. E. Amikacin: uses, resistance, and prospects for inhibition. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Serio, A. W.; Keepers, T.; Andrews, L.; Krause, K. M. Aminoglycoside revival: review of a historically important class of antimicrobials undergoing rejuvenation. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Aggen, J. B.; Armstrong, E. S.; Goldblum, A. A.; Dozzo, P.; Linsell, M. S.; Gliedt, M. J.; Hildebrandt, D. J.; Feeney, L. A.; Kubo, A.; Matias, R. D.; Lopez, S.; Gomez, M.; Wlasichuk, K. B.; Diokno, R.; Miller, G. H.; Moser, H. E. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother 2010, 54, 4636–4642. [Google Scholar] [CrossRef] [PubMed]
- Endimiani, A.; Hujer, K. M.; Hujer, A. M.; Armstrong, E. S.; Choudhary, Y.; Aggen, J. B.; Bonomo, R. A. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 2009, 53, 4504–4507. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Ejim, L.; Stogios, P. J.; Koteva, K.; Bordeleau, E.; Evdokimova, E.; Sieron, A. O.; Savchenko, A.; Serio, A. W.; Krause, K. M.; Wright, G. D. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis 2018, 4, 980–987. [Google Scholar] [CrossRef]
- Saravolatz, L. D.; Stein, G. E. Plazomicin: a new aminoglycoside. Clin Infect Dis 2020, 70, 704–709. [Google Scholar] [CrossRef]
- Lin, J.; Nishino, K.; Roberts, M. C.; Tolmasky, M.; Aminov, R. I.; Zhang, L. Mechanisms of antibiotic resistance. Front Microbiol 2015, 6, 34. [Google Scholar] [CrossRef]
- Clark, J. A.; Burgess, D. S. Plazomicin: a new aminoglycoside in the fight against antimicrobial resistance. Ther Adv Infect Dis 2020, 7, 2049936120952604. [Google Scholar] [CrossRef]
- Tang, H. J.; Lai, C. C. Plazomicin-associated Nephrotoxicity. Clin Infect Dis 2020, 71, 1130–1131. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Di Franco, S.; Donatiello, V.; Maffei, V.; Fittipaldi, C.; Fiore, M.; Coppolino, F.; Sansone, P.; Pace, M. C.; Passavanti, M. B. Plazomicin against multidrug-resistant bacteria: a scoping review. Life (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Macinga, D. R.; Rather, P. N. The chromosomal 2'-N-acetyltransferase of Providencia stuartii: physiological functions and genetic regulation. Front Biosci 1999, 4, D132–140. [Google Scholar] [CrossRef]
- Bassenden, A. V.; Dumalo, L.; Park, J.; Blanchet, J.; Maiti, K.; Arya, D. P.; Berghuis, A. M. Structural and phylogenetic analyses of resistance to next-generation aminoglycosides conferred by AAC(2') enzymes. Sci Rep 2021, 11, 11614. [Google Scholar] [CrossRef] [PubMed]
- Lin, D. L.; Tran, T.; Alam, J. Y.; Herron, S. R.; Ramirez, M. S.; Tolmasky, M. E. Inhibition of aminoglycoside 6'-N-acetyltransferase type Ib by zinc: reversal of amikacin resistance in Acinetobacter baumannii and Escherichia coli by a zinc ionophore. Antimicrob Agents Chemother 2014, 58, 4238–4241. [Google Scholar] [CrossRef] [PubMed]
- Chiem, K.; Fuentes, B. A.; Lin, D. L.; Tran, T.; Jackson, A.; Ramirez, M. S.; Tolmasky, M. E. Inhibition of aminoglycoside 6'-N-acetyltransferase type Ib-mediated amikacin resistance in Klebsiella pneumoniae by zinc and copper pyrithione. Antimicrob Agents Chemother 2015, 59, 5851–5853. [Google Scholar] [CrossRef] [PubMed]
- Chiem, K.; Hue, F.; Magallon, J.; Tolmasky, M. E. Inhibition of aminoglycoside 6'-N-acetyltransferase type Ib-mediated amikacin resistance by zinc complexed with clioquinol, an ionophore active against tumors and neurodegenerative diseases. Int J Antimicrob Agents 2018, 51, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Magallon, J.; Chiem, K.; Tran, T.; Ramirez, M. S.; Jimenez, V.; Tolmasky, M. E. Restoration of susceptibility to amikacin by 8-hydroxyquinoline analogs complexed to zinc. PLoS One 2019, 14, e0217602. [Google Scholar] [CrossRef] [PubMed]
- Magallon, J.; Vu, P.; Reeves, C.; Kwan, S.; Phan, K.; Oakley-Havens, C.; Ramirez, M. S.; Tolmasky, M. E. Amikacin in combination with zinc pyrithione prevents growth of a carbapenem-resistant/multidrug-resistant Klebsiella pneumoniae isolate. Int J Antimicrob Agents 2021, 58, 106442. [Google Scholar] [CrossRef]
- Reeves, C. M.; Magallon, J.; Rocha, K.; Tran, T.; Phan, K.; Vu, P.; Yi, Y.; Oakley-Havens, C. L.; Cedano, J.; Jimenez, V.; Ramirez, M. S.; Tolmasky, M. E. Aminoglycoside 6'-N-acetyltransferase type Ib [AAC(6')-Ib]-mediated aminoglycoside resistance: phenotypic conversion to susceptibility by silver ions. Antibiotics (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Ding, X.; Baca-DeLancey, R. R.; Rather, P. N. Role of SspA in the density-dependent expression of the transcriptional activator AarP in Providencia stuartii. FEMS Microbiol Lett 2001, 196, 25–29. [Google Scholar] [CrossRef]
- Thwaites, M.; Hall, D.; Shinabarger, D.; Serio, A. W.; Krause, K. M.; Marra, A.; Pillar, C. Evaluation of the bactericidal activity of plazomicin and comparators against multidrug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A. S. Emerging and reemerging infectious diseases: the perpetual challenge. Acad Med 2005, 80, 1079–1085. [Google Scholar] [CrossRef]
- Sprenger, M.; Fukuda, K. Antimicrobial resistance. New mechanisms, new worries. Science 2016, 351, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E. Rise in the prevalence of resistance to extended-spectrum cephalosporins in the USA, nursing homes and antibiotic prescribing in outpatient and inpatient settings. J Antimicrob Chemother 2021, 76, 2745–2747. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- Pierce, G. N.; Resch, C.; Mourin, M.; Dibrov, P.; Dibrov, E.; Ravandi, A. Bacteria and the growing threat of multidrug resistance for invasive cardiac interventions. Rev Cardiovasc Med 2022, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D. J.; Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis 2015, 15, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, A. K.; Boucher, H. W.; Fowler, V. G., Jr.; Jezek, A.; Outterson, K.; Greenberg, D. E. Antibiotic resistance in the patient with cancer: escalating challenges and paths forward. CA Cancer J Clin 2021, 71, 488–504. [Google Scholar] [CrossRef]
- WHO Ten threats to global health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 16 December 2022).
- Bush, K.; Bradford, P. A. Interplay between beta-lactamases and new beta-lactamase inhibitors. Nat Rev Microbiol 2019, 17, 295–306. [Google Scholar] [CrossRef]
- Li, Y.; Green, K. D.; Johnson, B. R.; Garneau-Tsodikova, S. Inhibition of aminoglycoside acetyltransferase resistance enzymes by metal salts. Antimicrob Agents Chemother 2015, 59, 4148–4156. [Google Scholar] [CrossRef]
- Bohlmann, L.; De Oliveira, D. M. P.; El-Deeb, I. M.; Brazel, E. B.; Harbison-Price, N.; Ong, C. Y.; Rivera-Hernandez, T.; Ferguson, S. A.; Cork, A. J.; Phan, M. D.; Soderholm, A. T.; Davies, M. R.; Nimmo, G. R.; Dougan, G.; Schembri, M. A.; Cook, G. M.; McEwan, A. G.; von Itzstein, M.; McDevitt, C. A.; Walker, M. J. Chemical synergy between ionophore PBT2 and zinc reverses antibiotic resistance. mBio 2018, 9. [Google Scholar] [CrossRef]
- Rather, P. N.; Orosz, E.; Shaw, K. J.; Hare, R.; Miller, G. Characterization and transcriptional regulation of the 2'-N-acetyltransferase gene from Providencia stuartii. J Bacteriol 1993, 175, 6492–6498. [Google Scholar] [CrossRef]
- Herisse, M.; Duverger, Y.; Martin-Verstraete, I.; Barras, F.; Ezraty, B. Silver potentiates aminoglycoside toxicity by enhancing their uptake. Mol Microbiol 2017, 105, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Morones-Ramirez, J. R.; Winkler, J. A.; Spina, C. S.; Collins, J. J. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med 2013, 5, 190ra181. [Google Scholar] [CrossRef]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and antibiotic, new facts to an old story. Antibiotics (Basel) 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A 1978, 75, 3737–3741. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S. N.; Chang, A. C.; Hsu, L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A 1972, 69, 2110–2114. [Google Scholar] [CrossRef]
- Haas, M. J.; Dowding, J. E. Aminoglycoside-modifying enzymes. Methods Enzymol 1975, 43, 611–628. [Google Scholar]
- Graham, F. L.; Smiley, J.; Russell, W. C.; Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977, 36, 59–74. [Google Scholar] [CrossRef]
- Tran, T.; Chiem, K.; Jani, S.; Arivett, B. A.; Lin, D. L.; Lad, R.; Jimenez, V.; Farone, M. B.; Debevec, G.; Santos, R.; Giulianotti, M.; Pinilla, C.; Tolmasky, M. E. Identification of a small molecule inhibitor of the aminoglycoside 6'-N-acetyltransferase type Ib [AAC(6')-Ib] using mixture-based combinatorial libraries. Int J Antimicrob Agents 2018, 51, 752–761. [Google Scholar] [CrossRef] [PubMed]
| Plazomicin (μg/ml) | Silver Acetate (μM) OD600 |
Sodium Acetate (μM) OD600 |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 0 | 8 | |
| 0 | 3.21 ± 0.02 | 3.09 ± 0.01 | 3.09 ± 0.02 | 3.18 ± 0.11 | 3.04 ± 0.06 | 3.12 ± 0.08 |
| 4 | 3.05 ± 0.08 | 2.86 ± 0.11 | 1.95 ± 0.01 | 0.12 ± 0.01 | 1.39 ± 0.03 | 1.34 ± 0.06 |
| 8 | 1.21 ± 0.01 | 1.26 ± 0.01 | 0.10 ± 0.03 | 0.02 ± 0 | 1.13 ± 0.02 | 1.08 ± 0.04 |
| Plazomicin (μg/ml) | Silver Acetate (μM) OD600 |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | ||
| 0 | 3.54 ± 0.01 | 3.48 ± 0.02 | 3.50 ± 0.07 | 3.35 ± 0.10 | |
| 4 | 1.71 ± 0.02 | 1.66 ± 0.09 | 0.95 ± 0.09 | 0.09 ± 0.07 | |
| 8 | 0.68 ± 0.06 | 0.24 ± 0.02 | 0.14 ± 0.06 | 0.03 ± 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





