1. INTRODUCTION
Within the past few decades, portable electronics have begun playing an increasing role in daily life, including microelectromechanical devices (MEMS) and nanoelectromechanical devices (NEMS) [
1,
2,
3,
4]. These devices, among many others, require a reliable energy storage and conversion device in order to function properly, the typical device being a battery. Many batteries are available for these applications, but the most promising proves to be lithium-ion batteries (LIBs), due mostly to their ability to provide high voltages and large energy storage densities [
5]. LIBs have found uses in a very wide range of applications as environmentally friendly electric vehicles [
6,
7] and rechargeable phone batteries [
8,
9,
10]. The main advantage of Lithium-ion batteries (Li-ion) is their high energy density. They have a long cycle of life. Li-ion does not suffer from the high self-discharge rate, and memory effect of nickel-cadmium (NiCd) and nickel metal hydride (NiMH) batteries. Unlike sealed lead acid (SLA) and NiCd, Li-ion batteries do not contain toxic heavy metals. The main disadvantage of Li-ion batteries is that they require careful attention to safety, overcharging, overheating, or short-circuiting a charged Li-ion battery can result in fire or explosion [
11].
The term “lithium-ion battery” refers to a diverse family of battery chemistries. All Li-ion batteries use a process known as intercalation, in which lithium ions are incorporated into the structure of the electrode material. Lithium ions move from the positive to the negative electrode during charging and from the negative to the positive electrode as the battery is discharged [
10,
12]. Most types of Li-ion batteries available today differ in the composition of their positive electrode (cathode). New materials for the negative elect3rode are also being developed, but few of these are now available on the market [
13].
The cathode is the positive electrode of the cell. There are many kinds of material are used to make cathode. Choosing any kind is based on the application that the cell will be used for. For instance, cost, safety, and density are mostly considered. Lithium Cobalt Oxide (LCO) is the most common material used in cathode fabrication. This type of material has many advantages which made it very common in commercial batteries these days, as instance: it has a high energy density, long cycle life, and does not suffer from high self-discharging. The anode is the negative electrode of the cell and there are many types of material used to make anode. As mentioned before, choosing any type of anode is based on the type of application that the cell will be used for. Typically, cost, safety, and density are the important characteristic that mostly considered. Graphite is the most common material using in these days due to many advantages especially the lower cost. There are many other anode materials that compete with graphite as Lithium Titanate (Li2TiO3), but they are still under research and not commercial yet. The difference between all-solid-state LIBs and conventional LIBs is the electrolyte; using a solid electrolyte offers many advantages, yet typically brings with it a loss of ionic conductivity, the key measure of electrolyte performance [
14,
15]. The coin battery cell guard usually is bigger in size than both the anode and cathode to prevent electrodes from touching each other.
In this paper, a LCO cathode is being fabricated and used to build for making two battery coin cells with different anodes and the same electrolyte. The two anodes are Lithium anode, and graphite anode, they were fabricated as well. The procedure steps are discussed in detail II. Section III discusses the experimental results of the batteries charging/discharging process and provides a comparison between the different fabricated batteries. Section IV is concluding the proposed work and highlights the future task and the improvement possibilities.
2. BATTERY FABRICATION
Experimentally, two battery coin cells have been fabricated in the lab. These two batteries have the same cathode, which is LCO, and the same electrolyte, but with different anodes. The two anodes are a graphite anode and a lithium anode. The anodes have been fabricated in the lab, as we will explain in detail in later sections.
2.1. LCO cathode fabrication
As we fabricate the cathode, first we specified the initial chemical components. Lithium Cobalt Oxide (LCO) cathode which is 95% of LCO, a pure carbon with a small percentage of 2%, and PVDF solution (which solved in (NMP) n-Methyl pyrrolidone solvent) with 5% of the weight. These three components need to be mixed very well, to do so we used a shaking machine to mix them for about 20 minutes. When the mix become very homogenous, we spread it on a very thin aluminum sheet as shown in
Figure 1 (weight will be considered later), then we left it in the dryer/vacuum machine for a night to dry and be coherent. The last step is rolling pieces of the aluminum sheet with cathode material on it (pieces are shown in
Figure 2) in a hot rolling machine, that had been set to 120 Co, several times until we get the shiniest and thinnest possible surface. At that moment, the cathode material is ready to be cut in a circle shape (circle shape shown below in
Figure 2) to use it for the cell.
2.2. Graphite anode fabrication
Graphite anode fabrication is very similar to Lithium Cobalt Oxide (LCO) cathode fabrication procedure. The total weight of the anode material is 1.5g which is enough to make more than one anode of the coin cell. We used pure graphite with pure carbon and PVDF solution, then mixed them using a shaking machine. As shown in
Figure 3 we spread the mix on a sheet of phosphor bronze instead of aluminum that was used in the LCO cathode. Next, the whole mixed slurry material was dried, rolled, and cut exactly as we did with the LCO cathode, then we used a circle shape to make the cell (shown in
Figure 4).
Capacity calculation:
The batteries capacity has been calculated as:
LCO/Lithium battery
LCO Cathode weight = 30.9 mg
LCO Cathode thickness = 0.04mm
Piece of aluminum sheet weight = 7 mg.
LCO weight= (30.9 - 7) × 93%=22.22 mg
LCO cathode capacity = (158 mAh/g) × (22.22) mg = 3.5 mAh
LCO/Graphite battery
LCO/Graphite battery
LCO Cathode weight = 26 mg
LCO Cathode thickness = 0.03 mm
Piece of aluminum sheet weight = 7 mg.
LCO weight= (26 - 7) X 93%=17.67 mg
LCO cathode capacity = (158 mAh/g) X (17.67) mg = 2.79 mAh
From the calculation, the LCO/lithium battery has a higher capacity than LCO/Graphite battery. The cathode has been used to calculate the capacity of batteries. The next section will show the experimental results of the charge/discharge of both batteries for purpose of comparison.
3. EXPERIMENTAL RESULTS
We tested both batteries with the same current rate (C-rate) to see their behavior.
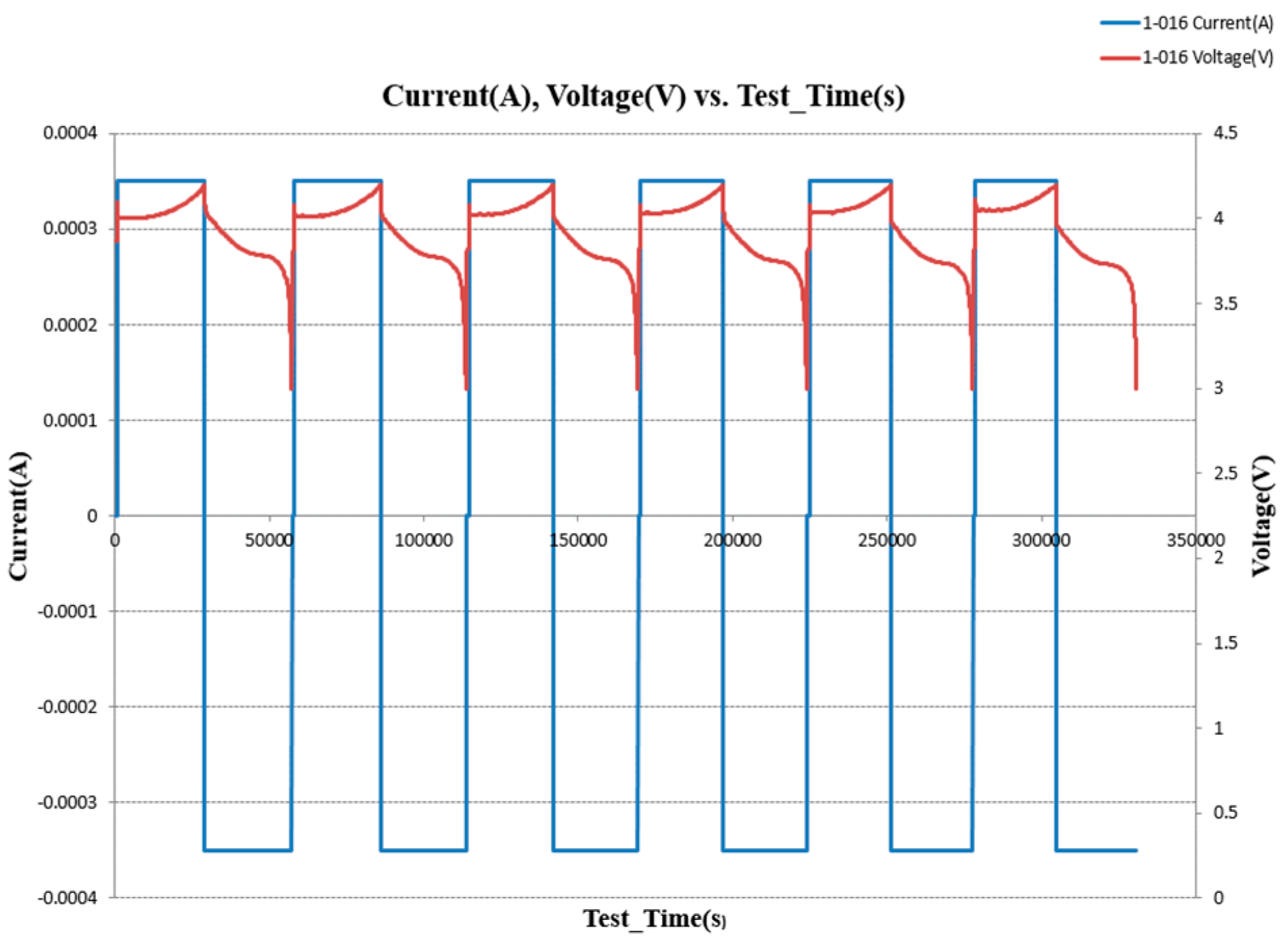
Figure 5 shows LCO/Lithium battery cycling for six cycles using 0.1 C-rate. The battery tested well with no issues and performed as expected. We noticed that LCO/lithium battery has charging and discharging cycles that took about 5600s. it does charge and discharge in the 3.4v to 4.3v range. By using 0.1 C-rate, the battery had the ability to charge and discharge very fast.
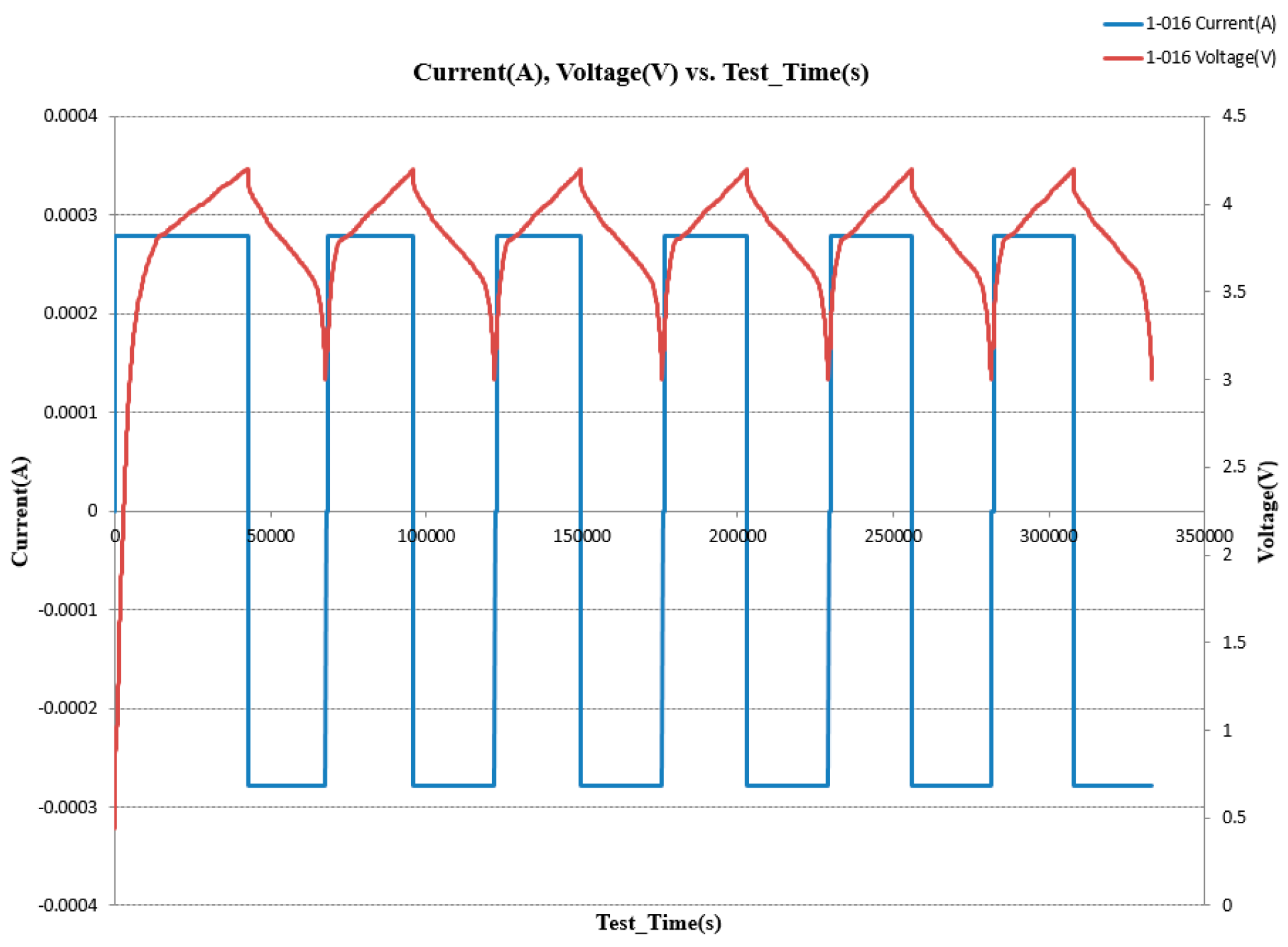
Figure 6 below shows LCO/Graphite battery cycling for six cycles using 0.1 C-rate. In the beginning, the battery did not work with 0.1 C-rate, so we started cycling with less than 0.1 until it reached the highest voltage which is 4.2 v. Then, it charged/discharged very normally as we expected.
4. Conclusion
Lithium-ion (Li-ion) batteries are the most commercially batteries used in the market due to their high energy density, lack of memory effect, and high charge and discharge rate capabilities. In this paper, we reviewed the types of Li-ion batteries. Then, we designed and fabricated a Li-ion battery and discussed the steps of fabrication. Two different cells with two different anodes and the same cathode have been modeled and fabricated then were used along with an electrolyte to construct coin batteries. Subsequently, batteries were charged and discharged using different C-rates. The chemical components have been measured and calculated carefully for high accuracy. Li-ion batteries have long cycle life and do not suffer from high discharge rates, and the main disadvantage is they require safety attention since overcharging a Li-ion battery can lead to a fire or explosion, and over-discharging can permanently damage the battery.
References
- S.-J. Lee, H.-K. Baik, S.-M. Lee. “An all-solid-state thin film battery using LISIPON electrolyte and Si–V negative electrode films,” Electrochemical Communication, vol. 5.1, pp. 32-35, January 2003. 20 January. [CrossRef]
- F. Albano, M. D. Chung, D. Blaauw, D. M. Sylvester, K. D. Wise, A. M. Sastry, “Design of an implantable power supply for an intraocular sensor, using POWER (power optimization for wireless energy requirements),” Journal of Power Sources, vol. 170.1, pp. 216-224, June 2007. 20 June. [CrossRef]
- Muntaser, A., Suleiman, A. A., & Lesewed, A. A. “Synthesis of Fuzzy Control System for Coupled Level Tanks”. 2015 International Conference on Information Science and Management Engineering (ICISME 2015) ISBN: 978-1-60595-303-8.
- Muntaser, A., & Buaossa, N. (2021). Coupled Tank Non-linear System; Modeling and Level Control using PID and Fuzzy Logic Techniques. arXiv preprint arXiv:2112.15506. arXiv:2112.15506. [CrossRef]
- J.-M. Tarascon, M. Armand, “Issues and challenges facing rechargeable lithium batteries,” Materials for Sustainable Energy, vol. 414, pp. 171- 179, 2010. [CrossRef]
- Muntaser, H. Elwarfalli, J. Kumar and G. Subramanyam, "Development of advanced energy storage system using fuzzy control," 2016 IEEE National Aerospace and Electronics Conference (NAECON) and Ohio Innovation Summit (OIS), 2016, pp. 179-182. [CrossRef]
- Elwarfalli, H., Abdalla Suleiman, M. M., & Muntaser, A. (2019, March). SCADA Control Levels for Two Tanks Process. In International Conference on Technical Sciences (ICST2019) (Vol. 6, p. 04).
- H. Kitaura, A. Hayashi, T. Ohtomo, S. Hama, M. Tatsumisago, “Fabrication of electrode–electrolyte interfaces in all-solid-state rechargeable lithium batteries by using a supercooled liquid state of the glassy electrolytes,” Journal of Material Chemistry, vol. 21, pp. 118−124, 2011. [CrossRef]
- H. Elwarfalli, A. Muntaser, J. Kumar and G. Subramanyam, "Design and implementation of PI controller for the hybrid energy system," 2016 IEEE National Aerospace and Electronics Conference (NAECON) and Ohio Innovation Summit (OIS), 2016, pp. 170-172. [CrossRef]
- A. Muntaser, H. Elwarfalli, A. Suleiman and G. Subramanyam, "Design and implementation of conventional (PID) and modern (Fuzzy logic) controllers for an energy storage system of hybrid electric vehicles," 2017 IEEE National Aerospace and Electronics Conference (NAECON), 2017, pp. 267-270. [CrossRef]
- Isaacson MJ, Hollandsworth RP, Giampaoli PJ, Linkowsky FA, Salim A, Teofilo VL. Advanced lithium ion battery charger. In: Fifteenth Annual Battery Conference on Applications and Advances, 2000. IEEE; 2000. pp. 193-198. [CrossRef]
- Zaghib K, Trudeau M, Guerfi A, Trottier J, Mauger A, Veillette R, Julien CM. New advanced cathode material: LiMnPO4 encapsulated with LiFePO4. Journal of Power Sources. 2012 April 15;204:177- 181.
- Patel P. Nanostructured Silicon Key to Better Batteries. IEEE Spectrum [Internet]. 2011 August [cited 2011 December 2]. Available from: http://spectrum.ieee.org/consumerelectronics/portabledevices/nanostructuredsilicon-key-to-betterbatteries.
- M. Nakayama, S. Wada, S. Kuroki, M. Nogami, “Factors affecting cyclic durability of all-solid-state lithium polymer batteries using poly(ethylene oxide)-based solid polymer electrolytes,” Energy and Environmental Science, vol. 3.12, pp. 1995−2002, 2010. [CrossRef]
- A. Patil, V. Patil, D. W. Shin, J.-W. Choi, D.-S. Paik, S.J. Yoon, “Issue and challenges facing rechargeable thin film lithium batteries,” Materials Research Bulletin, vol. 43.8-9, pp. 1913–1942, AugustSeptember 2008. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).