Submitted:
04 January 2023
Posted:
09 January 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results
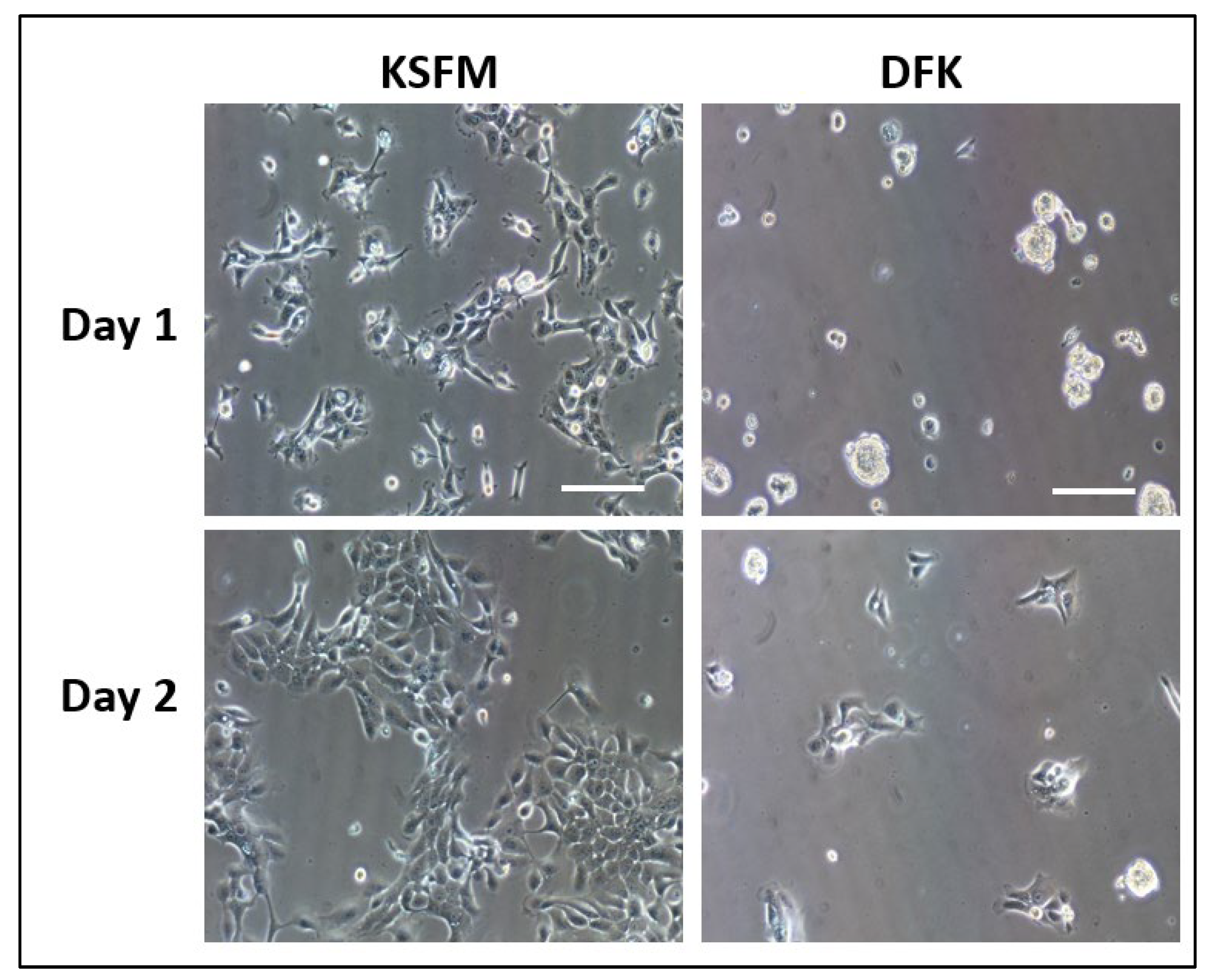
2.1. Seeding OKF6/TERT-2 cells in KSFM or DFK
2.2. OKF6/TERT-2 seeded in KSFM and switched to DFK
2.3. Confocal analysis of OKF6/TERT-2 morphology after growth in DFK and KSFM
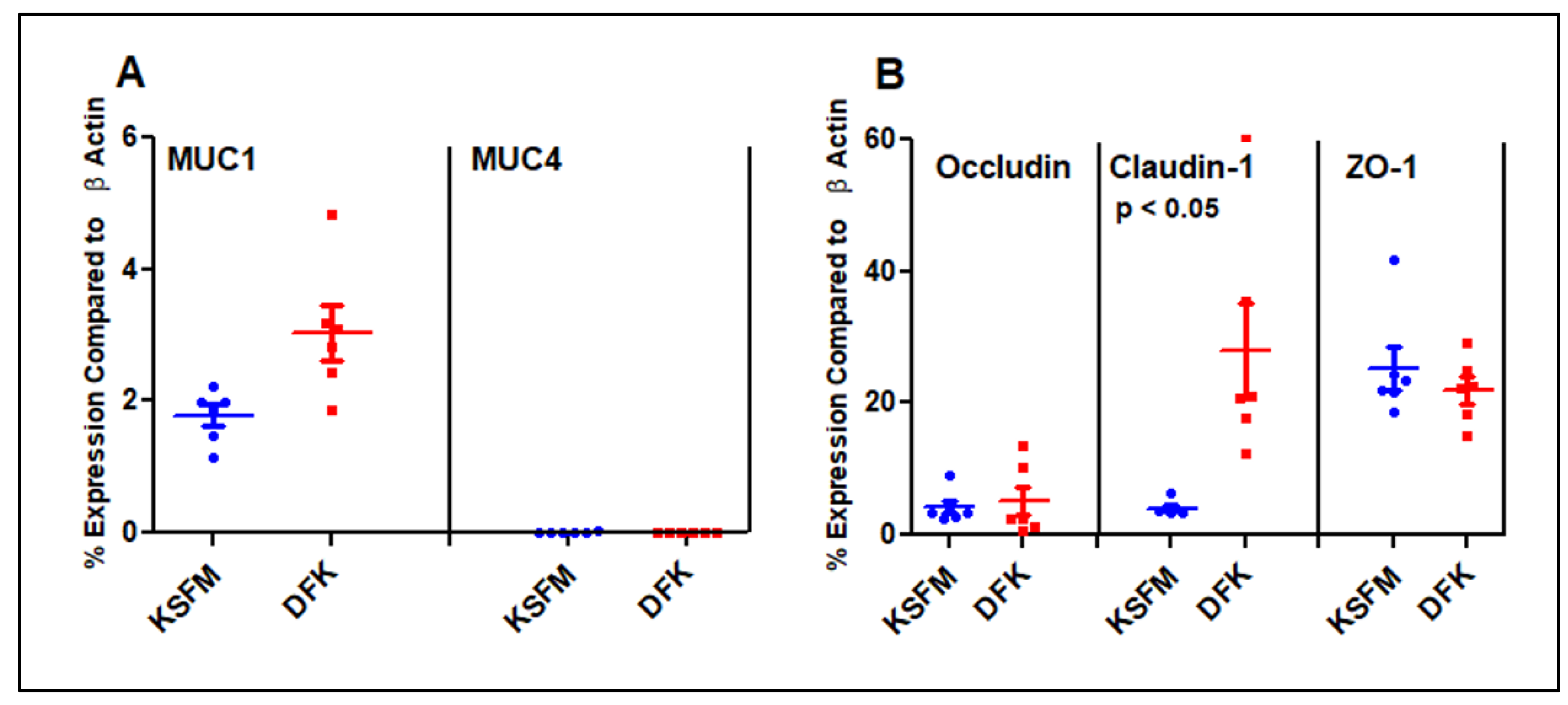
2.4. Expression of Mucins and Tight Junction genes after growth in DFK and KSFM
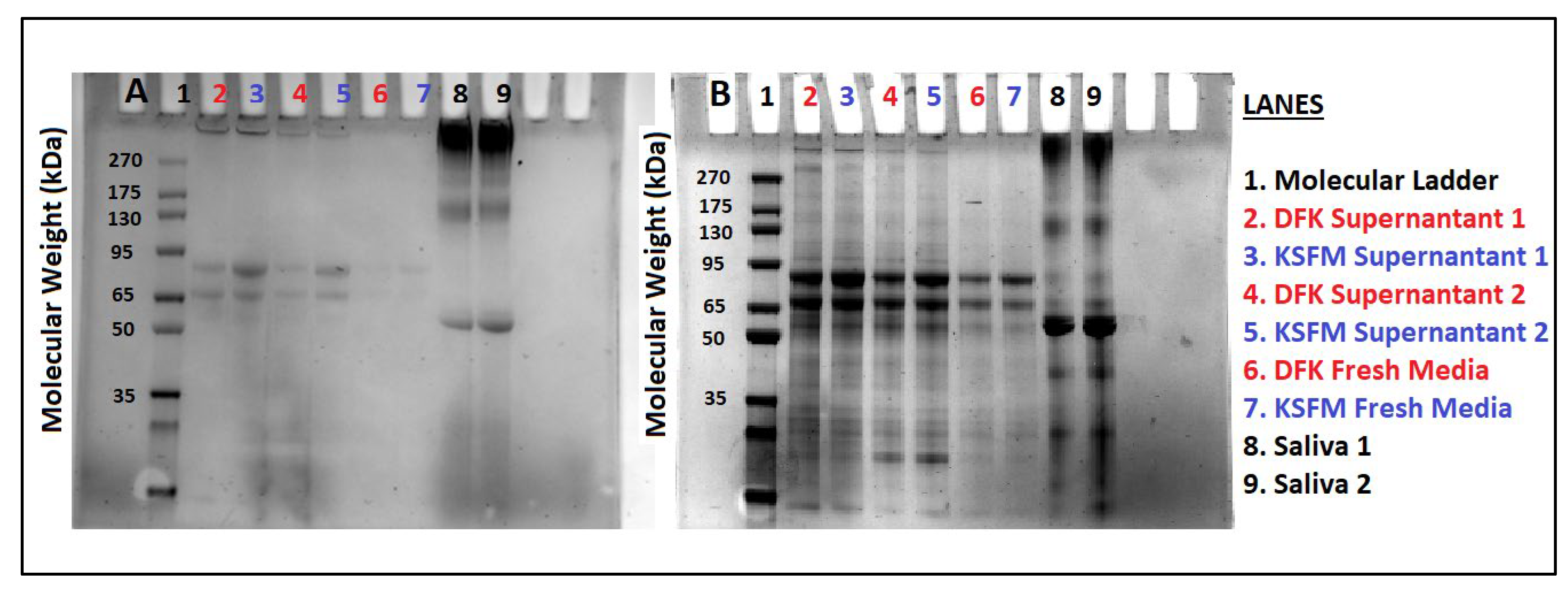
2.5. Release of Mucins after growth in DFK and KSFM
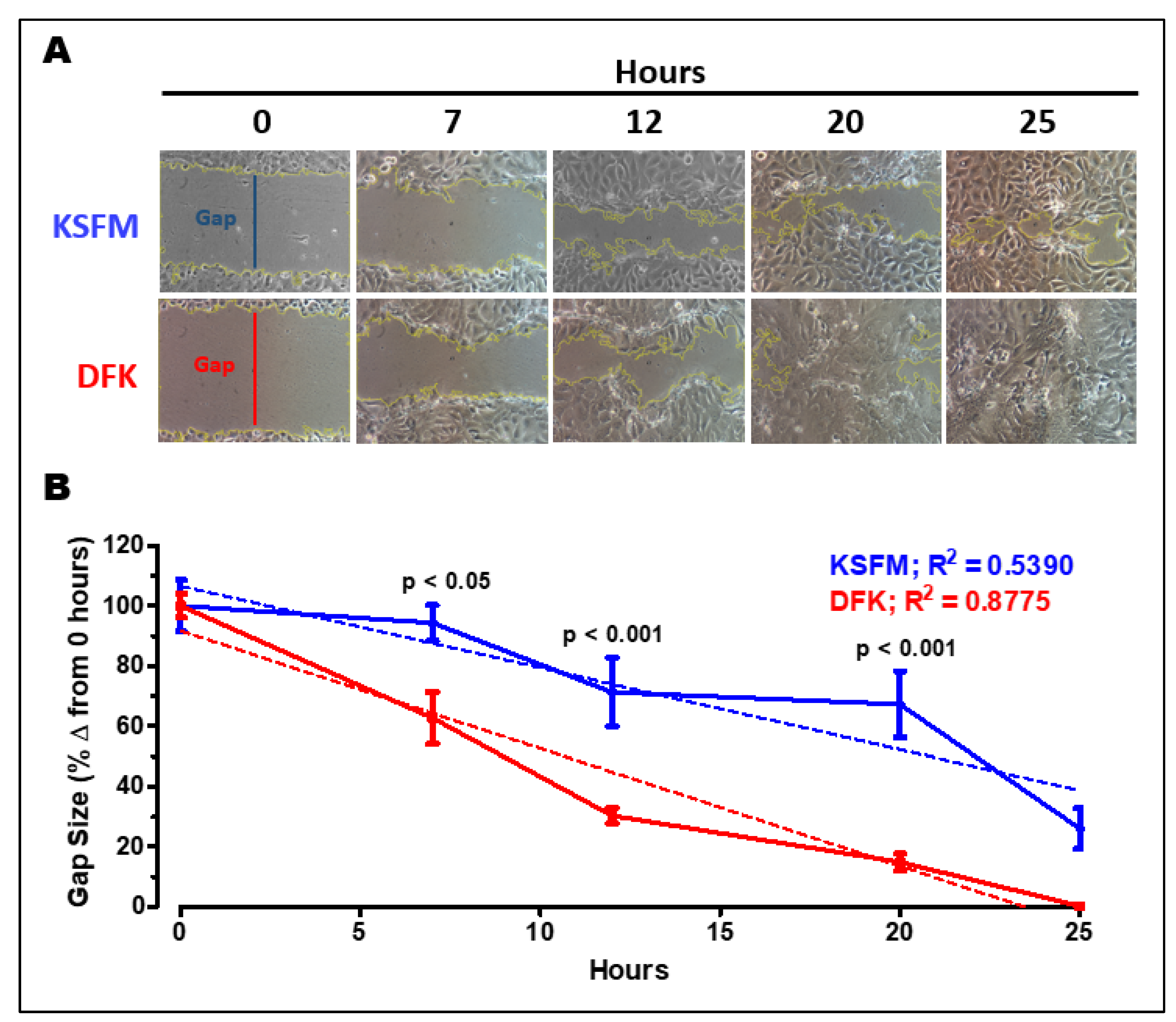
2.6. Wound healing assay in DFK and KSFM
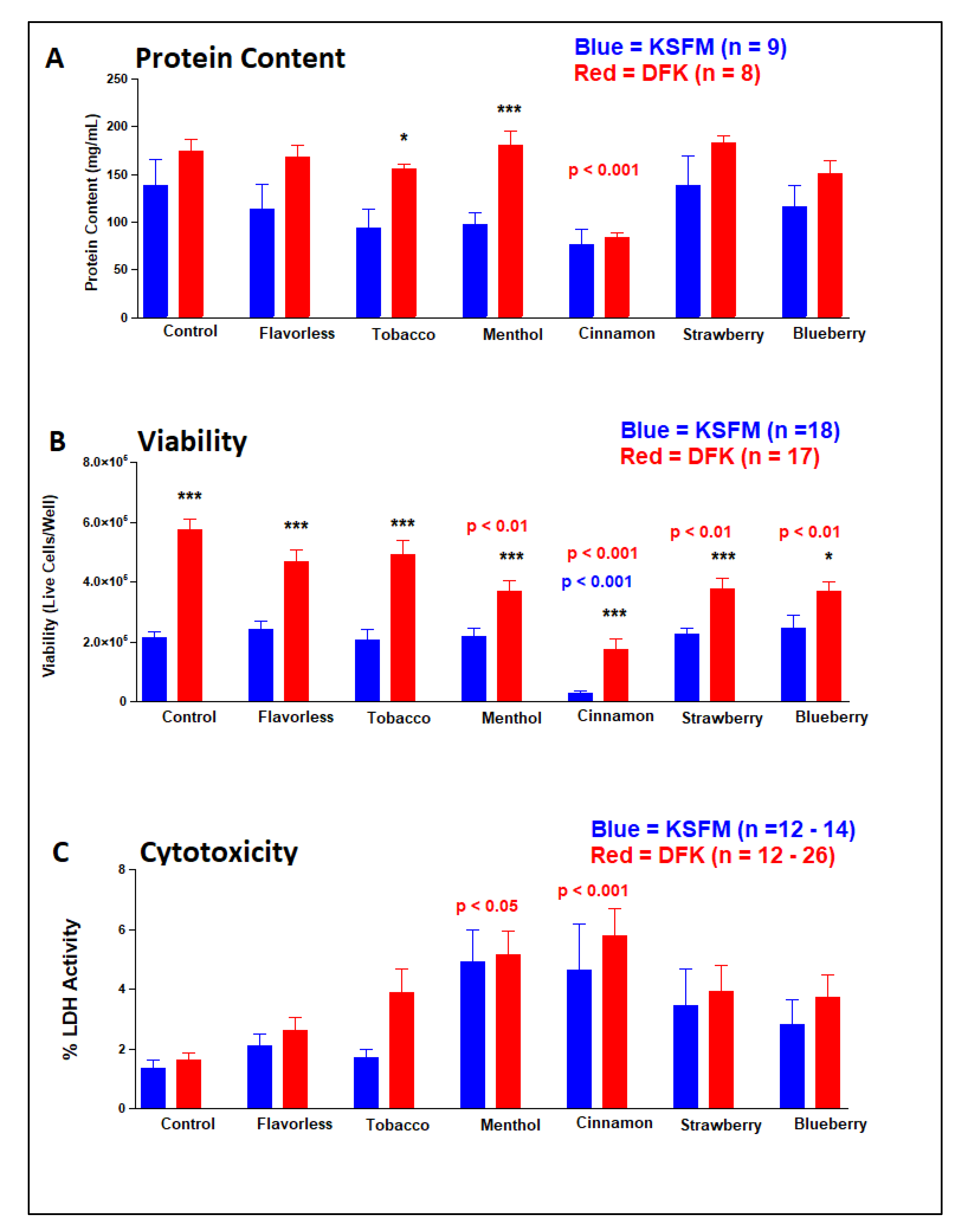
2.7. Effects of E-liquids ± flavors on OKF6/TERT cells after DFK and KSFM culturing
3. Discussion
4. Materials and Methods
4.1. Culture media
4.2. Cell culture, morphology and growth
4.4. Confocal Microscopy
4.5. Expression of mucins and tight junction genes
4.6. SDS-PAGE for released glycoproteins
4.7. Wound/Healing assay
4.8. Effects of E-liquid treatments: protein concentration, viability and cytotoxicity
4.9. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Louis, D.N.; Li, F.P.; Rheinwald, J.G. Human Keratinocytes That Express HTERT and Also Bypass a P16(INK4a)-Enforced Mechanism That Limits Life Span Become Immortal yet Retain Normal Growth and Differentiation Characteristics. Mol Cell Biol 2000, 20, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.D. Intestinal Transplantation: An Unexpected Journey. Robert, E. Gross Lecture. J Pediatr Surg 2014, 49, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Chingsuwanrote, P.; Yoosadiang, P.; Mekkriangkrai, D.; Ratchawong, T.; Buraphacheep, N.; Kijanukul, S.; Saekhow, S.; Pongpitchayadej, O.; Vongvachvasin, K.; et al. Partial Substitution of Glucose with Xylitol Suppressed the Glycolysis and Selectively Inhibited the Proliferation of Oral Cancer Cells. Nutr Cancer 2017, 69, 862–872. [Google Scholar] [CrossRef]
- Goessel, G.; Quante, M.; Hahn, W.C.; Harada, H.; Heeg, S.; Suliman, Y.; Doebele, M.; von Werder, A.; Fulda, C.; Nakagawa, H.; et al. Creating Oral Squamous Cancer Cells: A Cellular Model of Oral–Esophageal Carcinogenesis. Proc Natl Acad Sci U S A 2005, 102, 15599–15604. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, O.; Donegan, W.E.; Donovan, K.R.; Limonnik, V.; Azizkhan-Clifford, J. HSV-1 Hijacks the Host DNA Damage Response in Corneal Epithelial Cells through ICP4-Mediated Activation of ATM. Invest Ophthalmol Vis Sci 2020, 61, 39. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Alshaikh, A.; Kim, S.; Kim, J.; Chun, C.; Mehrazarin, S.; Lee, J.; Lux, R.; Kim, R.H.; Shin, K.H.; et al. Porphyromonas Gingivalis Impairs Oral Epithelial Barrier through Targeting GRHL2. J Dent Res 2019, 98, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.N.; Herzog, H.M.; James, M.G.; Cuadra, G.A. Effects of Oral Commensal Streptococci on Porphyromonas Gingivalis Invasion into Oral Epithelial Cells. Dent J (Basel) 2020, 8. [Google Scholar] [CrossRef]
- Rajagopalan, P.; Patel, K.; Jain, A.P.; Nanjappa, V.; Datta, K.K.; Subbannayya, T.; Mangalaparthi, K.K.; Kumari, A.; Manoharan, M.; Coral, K.; et al. Molecular Alterations Associated with Chronic Exposure to Cigarette Smoke and Chewing Tobacco in Normal Oral Keratinocytes. Cancer Biol Ther 2018, 19, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Patel, K.; Advani, J.; Subbannayya, T.; Rajagopalan, P.; Babu, N.; Solanki, H.; Bhandi, S.; Sidransky, D.; Chatterjee, A.; et al. Multiomic Analysis of Oral Keratinocytes Chronically Exposed to Shisha. J Oral Pathol Med 2019, 48, 284–289. [Google Scholar] [CrossRef]
- Nanjappa, V.; Renuse, S.; Sathe, G.J.; Raja, R.; Syed, N.; Radhakrishnan, A.; Subbannayya, T.; Patil, A.; Marimuthu, A.; Sahasrabuddhe, N.A.; et al. Chronic Exposure to Chewing Tobacco Selects for Overexpression of Stearoyl-CoA Desaturase in Normal Oral Keratinocytes. Cancer Biol Ther 2015, 16, 1593–1603. [Google Scholar] [CrossRef]
- Li, W.-C.; Ralphs, K.L.; Slack, J.M.W.; Tosh, D. Keratinocyte Serum-Free Medium Maintains Long-Term Liver Gene Expression and Function in Cultured Rat Hepatocytes by Preventing the Loss of Liver-Enriched Transcription Factors. Int J Biochem Cell Biol 2007, 39, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Cross, W.R.; Eardley, I.; Leese, H.J.; Southgate, J. A Biomimetic Tissue from Cultured Normal Human Urothelial Cells: Analysis of Physiological Function. American Journal of Physiology-Renal Physiology 2005, 289, F459–F468. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Pu, Q.; Zhang, Y.; Ma, Q.; Li, G.; Li, X. Expansion and Maintenance of Primary Corneal Epithelial Stem/Progenitor Cells by Inhibition of TGFβ Receptor I-Mediated Signaling. Exp Eye Res 2019, 182, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Perduns, R.; Volk, J.; Plum, M.; Gutzki, F.; Kaever, V.; Geurtsen, W. Effects of HEMA on Nrf2-Related Gene Expression Using a Newly Developed 3D Co-Culture Model of the Oral Mucosa. Dental Materials 2019, 35, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Peña-Oyarzún, D.; Silva, P.; Venegas, S.; Criollo, A.; Torres, V.A. Nuclear Accumulation of β-Catenin Is Associated with Endosomal Sequestration of the Destruction Complex and Increased Activation of Rab5 in Oral Dysplasia. FASEB J 2020, 34, 4009–4025. [Google Scholar] [CrossRef] [PubMed]
- Yiannis, C.; Huang, K.; Tran, A.N.; Zeng, C.; Dao, E.; Baselyous, O.; Mithwani, M.A.; Paolini, R.; Cirillo, N.; Yap, T.; et al. Protective Effect of Kava Constituents in an in Vitro Model of Oral Mucositis. J Cancer Res Clin Oncol 2020, 146, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Millhouse, E.; Jose, A.; Sherry, L.; Lappin, D.F.; Patel, N.; Middleton, A.M.; Pratten, J.; Culshaw, S.; Ramage, G. Development of an in Vitro Periodontal Biofilm Model for Assessing Antimicrobial and Host Modulatory Effects of Bioactive Molecules. BMC Oral Health 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.C.; Lin, A.L.; Cao, Z.; Zhao, X.-R.; Wu, L.-A.; Chen, S.; Sun, Y.; Yeh, C.-K. Anticandidal Activity and Biocompatibility of a Rechargeable Antifungal Denture Material. Oral Dis 2013, 19, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.; Leyhausen, G.; Geurtsen, W. Glutathione Level and Genotoxicity in Human Oral Keratinocytes Exposed to TEGDMA. J Biomed Mater Res B Appl Biomater 2012, 100, 391–399. [Google Scholar] [CrossRef]
- Volk, J.; Leyhausen, G.; Wessels, M.; Geurtsen, W. Reduced Glutathione Prevents Camphorquinone-Induced Apoptosis in Human Oral Keratinocytes. Dental Materials 2014, 30, 215–226. [Google Scholar] [CrossRef]
- Wessels, M.; Rimkus, J.; Leyhausen, G.; Volk, J.; Geurtsen, W. Genotoxic Effects of Camphorquinone and DMT on Human Oral and Intestinal Cells. Dental Materials 2015, 31, 1159–1168. [Google Scholar] [CrossRef]
- DMEM - Dulbecco’s Modified Eagle Medium - US. Available online: http://www.thermofisher.com/us/en/home/life-science/cell-culture/mammalian-cell-culture/classical-media/dmem.html (accessed on 8 January 2022).
- Almela, T.; Brook, I.M.; Moharamzadeh, K. Development of Three-Dimensional Tissue Engineered Bone-Oral Mucosal Composite Models. J Mater Sci Mater Med 2016, 27, 65. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.; AlQobaly, L.; Shaikh, Z.; Franklin, K.; Moharamzadeh, K. Implant Soft-Tissue Attachment Using 3D Oral Mucosal Models—A Pilot Study. Dent J (Basel) 2020, 8, 72. [Google Scholar] [CrossRef]
- Basso, F.G.; Cardoso, L.M.; Ribeiro, I.M.; Rizzi, E.; Pansani, T.N.; Hebling, J.; de Souza Costa, C.A. Influence of Bisphosphonates on Oral Implantology: Sodium Alendronate and Zoledronic Acid Enhance the Synthesis and Activity of Matrix Metalloproteinases by Gingival Fibroblasts Seeded on Titanium. Arch Oral Biol 2021, 127, 105134. [Google Scholar] [CrossRef]
- Ravosa, M.J.; Ning, J.; Liu, Y.; Stack, M.S. Bisphosphonate Effects on the Behaviour of Oral Epithelial Cells and Oral Fibroblasts. Arch Oral Biol 2011, 56, 491–498. [Google Scholar] [CrossRef]
- Fageeh, H.N.; Bhandi, S.; Mashyakhy, M.; Kahtani, A.A.; Badran, Z.; Mehta, D.; Fageeh, H.I.; Balaji, T.M.; Baeshen, H.A.; Varadarajan, S.; et al. Viability of Quercetin-Induced Dental Pulp Stem Cells in Forming Living Cellular Constructs for Soft Tissue Augmentation. J Pers Med 2021, 11, 430. [Google Scholar] [CrossRef]
- Omata, Y.; Lewis, J.B.; Rotenberg, S.; Lockwood, P.E.; Messer, R.L.W.; Noda, M.; Hsu, S.D.; Sano, H.; Wataha, J.C. Intra- and Extracellular Reactive Oxygen Species Generated by Blue Light. J Biomed Mater Res A 2006, 77, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Takai, Y. Recent Advances in Understanding Tight Junctions. Fac Rev 2021, 10, 18. [Google Scholar] [CrossRef]
- Anderson, J.M. Molecular Structure of Tight Junctions and Their Role in Epithelial Transport. News Physiol Sci 2001, 16, 126–130. [Google Scholar] [CrossRef]
- Dawes, C. Salivary Flow Patterns and the Health of Hard and Soft Oral Tissues. J Am Dent Assoc 2008, 139 Suppl, 18S–24S. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin Structure, Aggregation, Physiological Functions and Biomedical Applications. Current Opinion in Colloid & Interface Science 2006, 11, 164–170. [Google Scholar] [CrossRef]
- CREETH, J.M. CONSTITUENTS OF MUCUS AND THEIR SEPARATION. British Medical Bulletin 1978, 34, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.C.; Voynow, J.A. Respiratory Tract Mucin Genes and Mucin Glycoproteins in Health and Disease. Physiological Reviews 2006, 86, 245–278. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Frontiers in Immunology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Ueta, E.; Tanida, T.; Doi, S.; Osaki, T. Regulation of Candida Albicans Growth and Adhesion by Saliva. J Lab Clin Med 2000, 136, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Leone, C.W.; Oppenheim, F.G. Physical and Chemical Aspects of Saliva as Indicators of Risk for Dental Caries in Humans. J Dent Educ 2001, 65, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Gendler, S.J.; Spicer, A.P.; Lalani, E.N.; Duhig, T.; Peat, N.; Burchell, J.; Pemberton, L.; Boshell, M.; Taylor-Papadimitriou, J. Structure and Biology of a Carcinoma-Associated Mucin, MUC1. Am Rev Respir Dis 1991, 144, S42–47. [Google Scholar] [CrossRef]
- Gum, J.R. Mucin Genes and the Proteins They Encode: Structure, Diversity, and Regulation. Am J Respir Cell Mol Biol 1992, 7, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Sharma, S.; Gupta, A.; Gupta, N.; Singh, H.; Roychoudhury, A.; Mohanty, S.; Sen, S.; Nag, T.C.; Tandon, R. Molecular Characterization of Explant Cultured Human Oral Mucosal Epithelial Cells. Invest Ophthalmol Vis Sci 2011, 52, 9548–9554. [Google Scholar] [CrossRef] [PubMed]
- Supruniuk, K.; Czarnomysy, R.; Muszyńska, A.; Radziejewska, I. Anti-Cancer Effects of Pyrazole-Platinum(II) Complexes Combined with Anti-MUC1 Monoclonal Antibody versus Monotherapy in DLD-1 and HT-29 Colon Cancer Cells. Transl Oncol 2022, 18, 101348. [Google Scholar] [CrossRef]
- Abdelwhab, A.; Shaker, O.; Aggour, R.L. Expression of Mucin1 in Saliva in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders (Case Control Study). Oral Dis 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Liao, W.; Liang, T.; Liu, W.; Xie, J.; Wang, X.; Yang, P.; Lu, W.; Zhang, X. MUC1 Deficiency Induces the Nasal Epithelial Barrier Dysfunction via RBFOX3 Shortage Augments Ubiquitin-Proteasomal Degradation in Allergic Rhinitis Pathogenesis. Allergy 2022. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.P.; Palazzolo, D.L.; Cuadra, G.A. Mechanistic Effects of E-Liquids on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Flavoring Agents. Dent J (Basel) 2022, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Fischman, J.S.; Sista, S.; Lee, D.; Cuadra, G.A.; Palazzolo, D.L. Flavorless vs. Flavored Electronic Cigarette-Generated Aerosol and E-Liquid on the Growth of Common Oral Commensal Streptococci. Front Physiol 2020, 11, 585416. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, D.L.; Nelson, J.M.; Ely, E.A.; Crow, A.P.; Distin, J.; Kunigelis, S.C. The Effects of Electronic Cigarette (ECIG)-Generated Aerosol and Conventional Cigarette Smoke on the Mucociliary Transport Velocity (MTV) Using the Bullfrog (R. Catesbiana) Palate Paradigm. Front Physiol 2017, 8, 1023. [Google Scholar] [CrossRef] [PubMed]
- Ramenzoni, L.L.; Schneider, A.; Fox, S.C.; Meyer, M.; Meboldt, M.; Attin, T.; Schmidlin, P.R. Cytotoxic and Inflammatory Effects of Electronic and Traditional Cigarettes on Oral Gingival Cells Using a Novel Automated Smoking Instrument: An In Vitro Study. Toxics 2022, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Cátala-Valentín, A.R.; Almeda, J.; Bernard, J.N.; Cole, A.M.; Cole, A.L.; Moore, S.D.; Andl, C.D. E-Cigarette Aerosols Promote Oral, S. Aureus Colonization by Delaying an Immune Response and Bacterial Clearing. Cells 2022, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Negri, P.; Coniglio, M.; Bruscoli, S.; Di Michele, A.; Marchetti, M.C.; Valenti, C.; Gambelunghe, A.; Fanasca, L.; Billi, M.; et al. Heat-Not-Burn Tobacco (IQOS), Oral Fibroblasts and Keratinocytes: Cytotoxicity, Morphological Analysis, Apoptosis and Cellular Cycle. An in Vitro Study. J Periodontal Res 2021, 56, 917–928. [Google Scholar] [CrossRef]
- Tellez, C.S.; Juri, D.E.; Phillips, L.M.; Do, K.; Yingling, C.M.; Thomas, C.L.; Dye, W.W.; Wu, G.; Kishida, S.; Kiyono, T.; et al. Cytotoxicity and Genotoxicity of E-Cigarette Generated Aerosols Containing Diverse Flavoring Products and Nicotine in Oral Epithelial Cell Lines. Toxicol Sci 2021, 179, 220–228. [Google Scholar] [CrossRef]
- Beklen, A.; Uckan, D. Electronic Cigarette Liquid Substances Propylene Glycol and Vegetable Glycerin Induce an Inflammatory Response in Gingival Epithelial Cells. Hum Exp Toxicol 2021, 40, 25–34. [Google Scholar] [CrossRef]
- Stratified Epithelium. Available online: https://www.kenhub.com/en/library/anatomy/stratified-epithelium (accessed on 16 June 2022).
- Moffatt-Jauregui, C.E.; Robinson, B.; de Moya, A.V.; Brockman, R.D.; Roman, A.V.; Cash, M.N.; Culp, D.J.; Lamont, R.J. Establishment and Characterization of a Telomerase Immortalized Human Gingival Epithelial Cell Line. J Periodontal Res 2013, 48, 10–1111. [Google Scholar] [CrossRef] [PubMed]
- Oda, D.; Watson, E. Human Oral Epithelial Cell Culture I. Improved Conditions for Reproducible Culture in Serum-Free Medium. In Vitro Cell Dev Biol 1990, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Morino, T.; Takagi, R.; Yamamoto, K.; Kojima, H.; Yamato, M. Explant Culture of Oral Mucosal Epithelial Cells for Fabricating Transplantable Epithelial Cell Sheet. Regen Ther 2018, 10, 36–45. [Google Scholar] [CrossRef]
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Frontiers in Immunology 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kasai, Y.; Sugiyama, H.; Takagi, R.; Kondo, M.; Owaki, T.; Namiki, H.; Okano, T.; Takeda, N.; Yamato, M. Brush Biopsy of Human Oral Mucosal Epithelial Cells as a Quality Control of the Cell Source for Fabrication of Transplantable Epithelial Cell Sheets for Regenerative Medicine. Regenerative Therapy 2016, 4, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pelaez-Prestel, H.F.; Sanchez-Trincado, J.L.; Lafuente, E.M.; Reche, P.A. Immune Tolerance in the Oral Mucosa. Int J Mol Sci 2021, 22, 12149. [Google Scholar] [CrossRef]
- Forslind, B. Quantitative X-Ray Microanalysis of Skin. Particle Probe Evaluation of the Skin Barrier Function. Acta Derm Venereol Suppl (Stockh) 1987, 134, 1–8. [Google Scholar]
- Forslind, B.; Lindberg, M.; Malmqvist, K.G.; Pallon, J.; Roomans, G.M.; Werner-Linde, Y. Human Skin Physiology Studied by Particle Probe Microanalysis. Scanning Microsc 1995, 9, 1011–1025. [Google Scholar]
- Forslind, B.; Lindberg, M.; Roomans, G.M.; Pallon, J.; Werner-Linde, Y. Aspects on the Physiology of Human Skin: Studies Using Particle Probe Analysis. Microsc Res Tech 1997, 38, 373–386. [Google Scholar] [CrossRef]
- Pallon, J.; Malmqvist, K.G.; Werner-Linde, Y.; Forslind, B. Pixe Analysis of Pathological Skin with Special Reference to Psoriasis and Atopic Dry Skin. Cell Mol Biol (Noisy-le-grand) 1996, 42, 111–118. [Google Scholar]
- Leinonen, P.T.; Hägg, P.M.; Peltonen, S.; Jouhilahti, E.-M.; Melkko, J.; Korkiamäki, T.; Oikarinen, A.; Peltonen, J. Reevaluation of the Normal Epidermal Calcium Gradient, and Analysis of Calcium Levels and ATP Receptors in Hailey–Hailey and Darier Epidermis. J Invest Dermatol 2009, 129, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, T.R.; Shi, J.; Chen, D.; Trivedi, H.M.; Chen, L. Differential Expression and Function of Bicellular Tight Junctions in Skin and Oral Wound Healing. Int J Mol Sci 2020, 21, E2966. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Harty, D.; Commandeur, Z.; Hunter, N. Binding of Streptococcus Gordonii to Oral Epithelial Monolayers Increases Paracellular Barrier Function. Microb Pathog 2013, 56, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Alshaikh, A.; Kim, S.; Kim, J.; Chun, C.; Mehrazarin, S.; Lee, J.; Lux, R.; Kim, R.H.; Shin, K.H.; et al. Porphyromonas Gingivalis Impairs Oral Epithelial Barrier through Targeting GRHL2. J Dent Res 2019, 98, 1150–1158. [Google Scholar] [CrossRef]
- Lagha, A.B.; Groeger, S.; Meyle, J.; Grenier, D. Green Tea Polyphenols Enhance Gingival Keratinocyte Integrity and Protect against Invasion by Porphyromonas Gingivalis. Pathog Dis 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, P.; Liu, Z.-H.; Ye, P. Analysis of Differential Expression of Tight Junction Proteins in Cultured Oral Epithelial Cells Altered by Porphyromonas Gingivalis, Porphyromonas Gingivalis Lipopolysaccharide, and Extracellular Adenosine Triphosphate. Int J Oral Sci 2018, 10, e8. [Google Scholar] [CrossRef] [PubMed]
- Rosace, D.; Gomez-Casado, C.; Fernandez, P.; Perez-Gordo, M.; Dominguez, M.D.C.; Vega, A.; Belver, M.T.; Ramos, T.; Vega, F.; Marco, G.; et al. Profilin-Mediated Food-Induced Allergic Reactions Are Associated with Oral Epithelial Remodeling. J Allergy Clin Immunol 2019, 143, 681–690.e1. [Google Scholar] [CrossRef] [PubMed]
- Najarro, K.M.; Boe, D.M.; Walrath, T.M.; Mullen, J.E.; Paul, M.T.; Frankel, J.H.; Hulsebus, H.J.; Idrovo, J.-P.; McMahan, R.H.; Kovacs, E.J. Advanced Age Exacerbates Intestinal Epithelial Permeability after Burn Injury in Mice. Experimental Gerontology 2022, 158, 111654. [Google Scholar] [CrossRef] [PubMed]
- Gendler, S.J. MUC1, the Renaissance Molecule. J Mammary Gland Biol Neoplasia 2001, 6, 339–353. [Google Scholar] [CrossRef]
- Wreschner, D.H.; McGuckin, M.A.; Williams, S.J.; Baruch, A.; Yoeli, M.; Ziv, R.; Okun, L.; Zaretsky, J.; Smorodinsky, N.; Keydar, I.; et al. Generation of Ligand-Receptor Alliances by “SEA” Module-Mediated Cleavage of Membrane-Associated Mucin Proteins. Protein Sci 2002, 11, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Levitin, F.; Stern, O.; Weiss, M.; Gil-Henn, C.; Ziv, R.; Prokocimer, Z.; Smorodinsky, N.I.; Rubinstein, D.B.; Wreschner, D.H. The MUC1 SEA Module Is a Self-Cleaving Domain. J Biol Chem 2005, 280, 33374–33386. [Google Scholar] [CrossRef]
- Parry, S.; Silverman, H.S.; McDermott, K.; Willis, A.; Hollingsworth, M.A.; Harris, A. Identification of MUC1 Proteolytic Cleavage Sites in Vivo. Biochem Biophys Res Commun 2001, 283, 715–720. [Google Scholar] [CrossRef]
- Arcasoy, S.M.; Latoche, J.; Gondor, M.; Watkins, S.C.; Henderson, R.A.; Hughey, R.; Finn, O.J.; Pilewski, J.M. MUC1 and Other Sialoglycoconjugates Inhibit Adenovirus-Mediated Gene Transfer to Epithelial Cells. Am J Respir Cell Mol Biol 1997, 17, 422–435. [Google Scholar] [CrossRef]
- Walters, R.W.; Pilewski, J.M.; Chiorini, J.A.; Zabner, J. Secreted and Transmembrane Mucins Inhibit Gene Transfer with AAV4 More Efficiently than AAV5. J Biol Chem 2002, 277, 23709–23713. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Hyun, S.W.; Kim, B.T.; Zhang, X.G.; Lee, D.I.; Rowland, S.; Kim, K.C. Muc1 Mucins on the Cell Surface Are Adhesion Sites for Pseudomonas Aeruginosa. Am J Physiol Lung Cell Mol Physiol 2001, 280, L181–187. [Google Scholar] [CrossRef]
- Lindén, S.K.; Sheng, Y.H.; Every, A.L.; Miles, K.M.; Skoog, E.C.; Florin, T.H.J.; Sutton, P.; McGuckin, M.A. MUC1 Limits Helicobacter Pylori Infection Both by Steric Hindrance and by Acting as a Releasable Decoy. PLoS Pathog 2009, 5, e1000617. [Google Scholar] [CrossRef]
- Lima, B.P.; Davies, J.R.; Wickström, C.; Johnstone, K.F.; Hall, J.W.; Svensater, G.; Herzberg, M.C. Streptococcus Gordonii Poised for Glycan Feeding through a MUC5B-Discriminating, Lipoteichoic Acid-Mediated Outside-In Signaling Circuit. J Bacteriol 2022, 204, e0011822. [Google Scholar] [CrossRef]
- Culp, D.J.; Robinson, B.; Cash, M.N.; Bhattacharyya, I.; Stewart, C.; Cuadra-Saenz, G. Salivary Mucin 19 Glycoproteins: Innate Immune Functions in Streptococcus Mutans-Induced Caries in Mice and Evidence for Expression in Human Saliva. J Biol Chem 2015, 290, 2993–3008. [Google Scholar] [CrossRef]
- Frenkel, E.S.; Ribbeck, K. Salivary Mucins Protect Surfaces from Colonization by Cariogenic Bacteria. Appl Environ Microbiol 2015, 81, 332–338. [Google Scholar] [CrossRef]
- Ling, L.-Q.R.; Lin, Z.; Paolini, R.; Farah, C.S.; McCullough, M.; Lim, M.A.W.T.; Celentano, A. Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro. Biology (Basel) 2022, 11, 596. [Google Scholar] [CrossRef]
- Dennis, M.; Wang, G.; Luo, J.; Lin, Y.; Dohadwala, M.; Sidell, D.; DeConde, A.; Abemayor, E.; Elashoff, D.A.; Sharma, S.; et al. Snail Controls the Mesenchymal Phenotype and Drives Erlotinib Resistance in Oral Epithelial and HNSCC Cells. Otolaryngol Head Neck Surg 2012, 147, 726–732. [Google Scholar] [CrossRef]
- Shaikh, Z.N.; Alqahtani, A.; Almela, T.; Franklin, K.; Tayebi, L.; Moharamzadeh, K. Effects of Electronic Cigarette Liquid on Monolayer and 3D Tissue-Engineered Models of Human Gingival Mucosa. J Adv Periodontol Implant Dent 2019, 11, 54–62. [Google Scholar] [CrossRef]
- Alanazi, H.; Semlali, A.; Chmielewski, W.; Rouabhia, M. E-Cigarettes Increase Candida Albicans Growth and Modulate Its Interaction with Gingival Epithelial Cells. International Journal of Environmental Research and Public Health 2019, 16, 294. [Google Scholar] [CrossRef]
- Catala-Valentin, A.; Bernard, J.N.; Caldwell, M.; Maxson, J.; Moore, S.D.; Andl, C.D. E-Cigarette Aerosol Exposure Favors the Growth and Colonization of Oral Streptococcus Mutans Compared to Commensal Streptococci. Microbiol Spectr 10, e02421-21. [CrossRef]
- Cátala-Valentín, A.R.; Almeda, J.; Bernard, J.N.; Cole, A.M.; Cole, A.L.; Moore, S.D.; Andl, C.D. E-Cigarette Aerosols Promote Oral S. Aureus Colonization by Delaying an Immune Response and Bacterial Clearing. Cells 2022, 11, 773. [Google Scholar] [CrossRef]
- Yessentayeva, S.Y.; Orakbay, L.Z.; Adilhanova, A.; Yessimov, N. Approaches to the Use of Stem Cells in Regenerative Medicine. Anal Biochem 2022, 645, 114608. [Google Scholar] [CrossRef]
- Moysidou, C.-M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front Bioeng Biotechnol 2020, 8, 620962. [Google Scholar] [CrossRef]
- Klausner, M.; Handa, Y.; Aizawa, S. In Vitro Three-Dimensional Organotypic Culture Models of the Oral Mucosa. In Vitro Cell Dev Biol Anim 2021, 57, 148–159. [Google Scholar] [CrossRef]
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of Soft Tissue Repair: Gingival Epithelium in Wound Healing and Attachment to the Tooth and Abutment Surface. Eur Cell Mater 2019, 38, 63–78. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa. Int J Oral Sci 2020, 12, 1–5. [Google Scholar] [CrossRef]
- Daniell, H.; Nair, S.K.; Esmaeili, N.; Wakade, G.; Shahid, N.; Ganesan, P.K.; Islam, M.R.; Shepley-McTaggart, A.; Feng, S.; Gary, E.N.; et al. Debulking SARS-CoV-2 in Saliva Using Angiotensin Converting Enzyme 2 in Chewing Gum to Decrease Oral Virus Transmission and Infection. Molecular Therapy 2021, 0. [Google Scholar] [CrossRef]
- Moller, H.J.; Poulsen, J.H. Improved Method for Silver Staining of Glycoproteins in Thin Sodium Dodecyl Sulfate Polyacrylamide Gels. Analytical Biochemistry 1995, 226, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ Ecosystem: An Open Platform for Biomedical Image Analysis. Mol Reprod Dev 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




