Submitted:
08 January 2023
Posted:
09 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
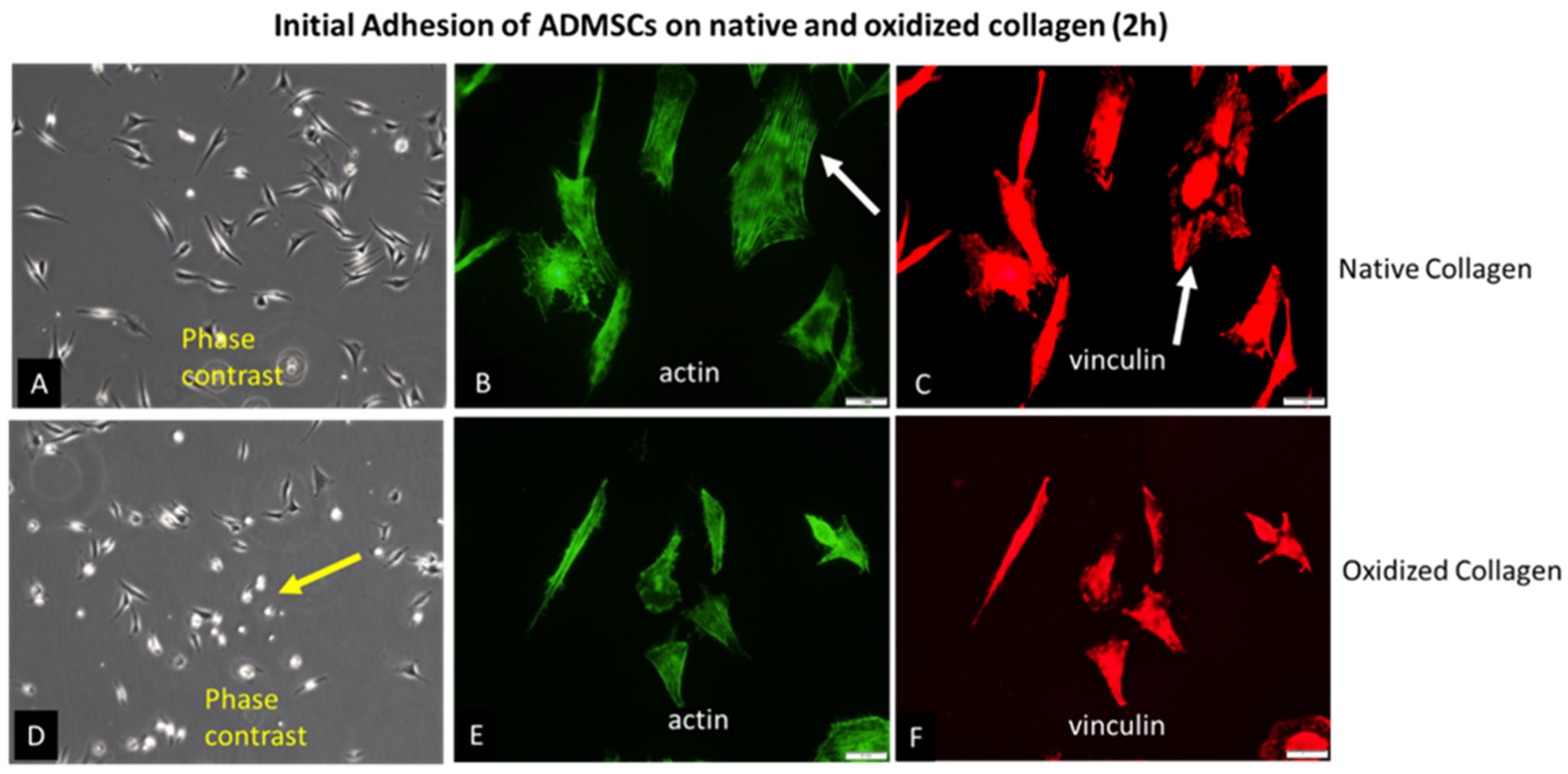
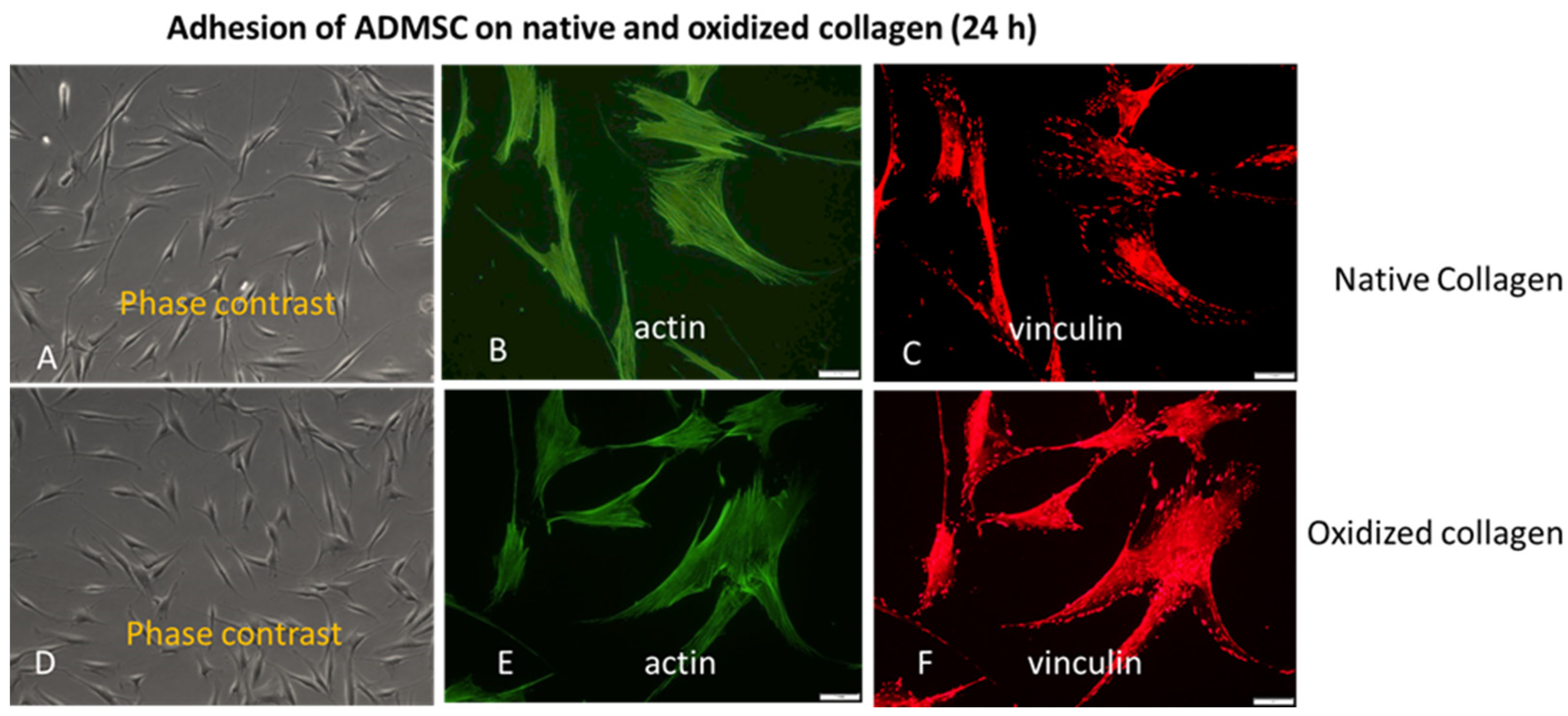
2.1. Initial Cell Attachment
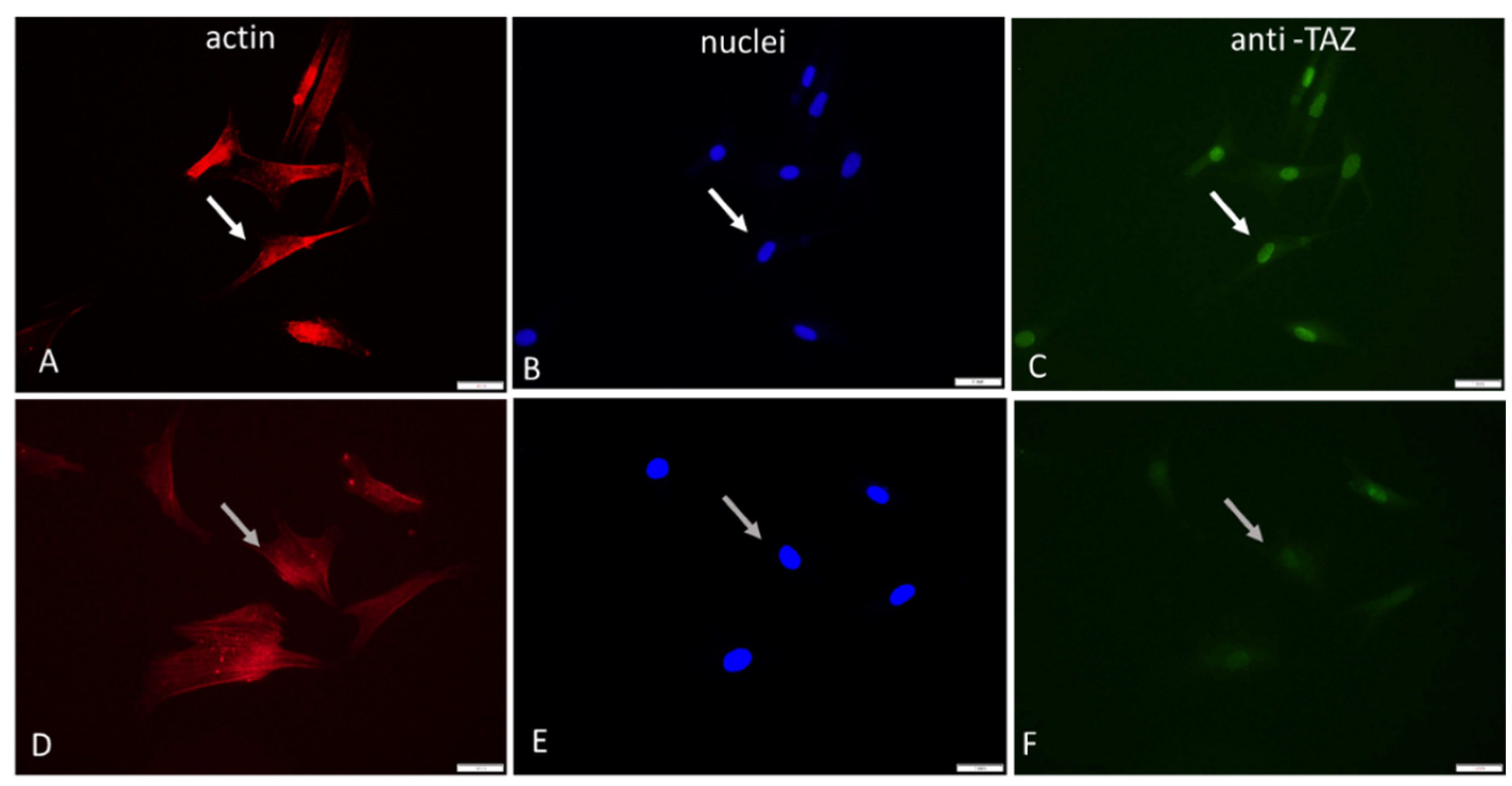
2.2. YAP/TAZ Signalling Events
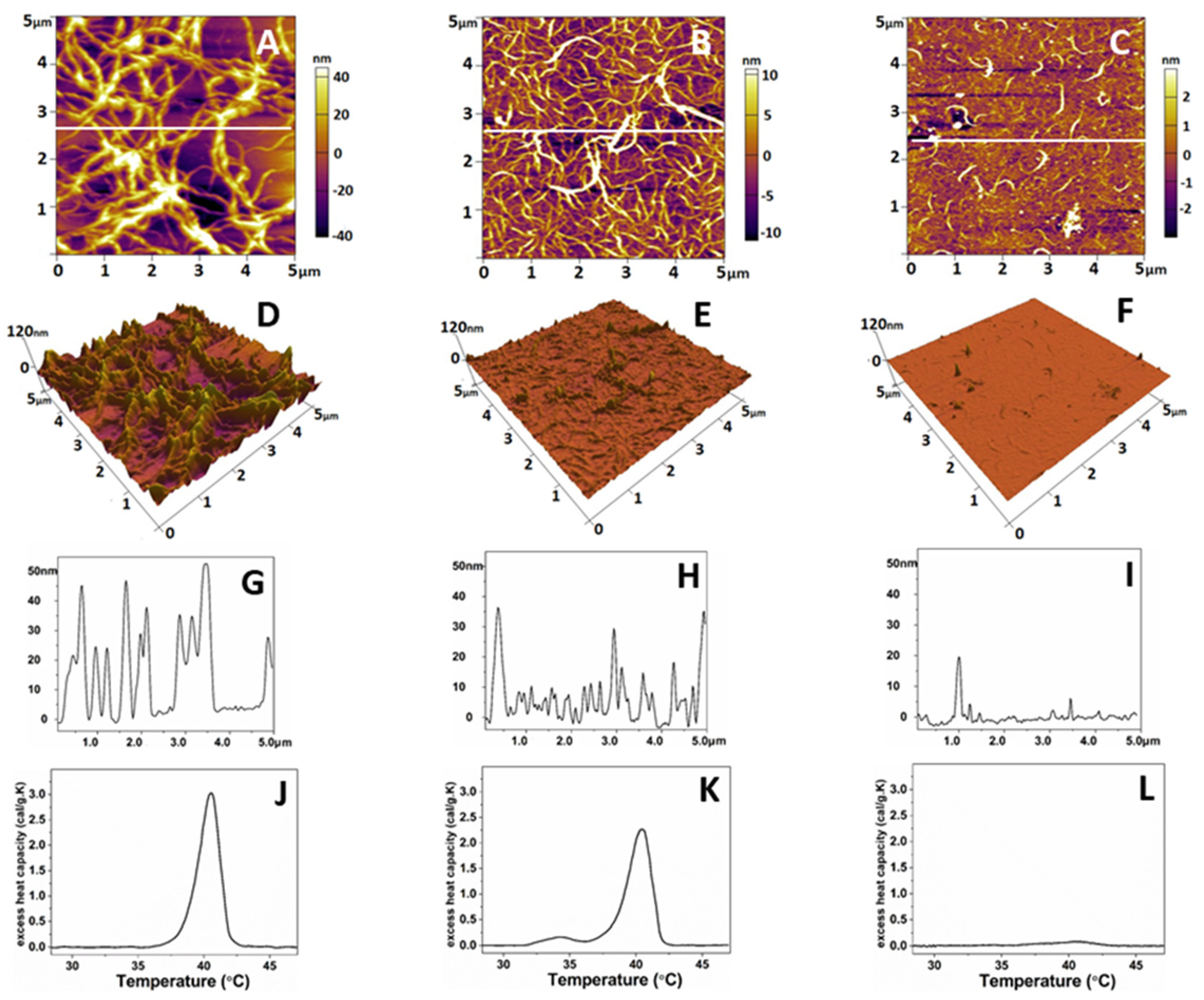
2.3. Comparative AFM Study
3. Discussion
4. Materials and Methods
4.1. Collagen Preparation
4.2. Collagen Oxidation Procedure
4.3. Cells
4.4. Morphological Study
4.5. Image Analysis
4.5.1. Quantitative Analysis of Raw Format Images by ImageJ
4.5.2. Quantification of Overall Morphological Parameters
4.5.3. YAP/TAZ Signaling
4.5.4. Quantification of Focal Adhesions (FA)
4.6. AFM Studies
4.7. DSC Measurements
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burridge K, Monaghan Benson E, Graham DM. Mechanotransduction: from the cell surface to the nucleus via RhoA. Phil. Trans. R. Soc. 2019. B 374: 20180229. [CrossRef]
- Yamashiroa Y, Quoc Thangb B, Ramireza K, Jae Shina S, Kohatae T, Ohataf S, Anh Vu Nguyena T, Ohtsukie S, Nagayamaf K, Yanagisawaa H Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. PNAS, 2020, vol. 117, no. 18. [CrossRef]
- Vining KH and Mooney DJ: Mechanical forces direct stem cell behavior in development and regeneration. Nat Rev Mol Cell Biol, 2017, 18: 728-742,. [CrossRef]
- Halder G, Dupont S and Piccolo S: Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 13: 591-600, 2012. [CrossRef]
- Humphrey J. D., Dufresne E. R., Schwartz M. A., Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014). [CrossRef]
- Engler AJ, Sen S, Sweeney HL and Discher DE: Matrix elasticity directs stem cell lineage specification. Cell 126: 677-689, 2006. [CrossRef]
- Li D, Zhou J, Chowdhury F, Cheng J, Wang N and Wang F: Role of mechanical factors in fate decisions of stem cells, Regen Med. 2011 Mar; 6(2): 229–240. [CrossRef]
- Lee JH, Park HK and Kim KS: Intrinsic and extrinsic mechanical properties related to the differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 473: 752-757, 2016. [CrossRef]
- Hou Y, Xie W, Yu L, Cuellar Camacho L, Nie C, Zhang M, Haag R, Wei Q. Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells, Small, 16 (10). [CrossRef]
- Wang Y, Wang G, Luo X, Qiu J and Tang C: Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns 38: 414 420, 2012. [CrossRef]
- Shalabi MM, Gortemaker A, Van’t Hof M A, Jansen J A, Creugers N H J. Implant surface roughness and bone healing: a systematic review J Dent Res. 2006 Jun;85(6):496-500. [CrossRef]
- Matos GRM (2021) Surface Roughness of Dental Implant and Osseointegration Maxillofac Oral Surg. 2021 Mar; 20(1): 1–4. [CrossRef]
- Llopis-Hernandez V, Rico P, Moratal D, Altankov G and Salmeron-Sanchez M (2013) Role of Material-Driven Fibronectin Fibrillogenesis, Acta Biomaterialia, 77, pp. 74-84. [CrossRef]
- Klein E. A. et al., Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr. Biol. 19, 1511–1518 (2009). 4. [CrossRef]
- Nedjari S, Awaja F & Altankov G (2017) Three Dimensional Honeycomb Patterned Fibrinogen Based Nanofibers Induce Substantial Osteogenic Response of Mesenchymal Stem Cells, Scientific Reports, 7, 15947. [CrossRef]
- Kim D., Provenzano P.P, Smith C. L., Levchenko A., Matrix nanotopography as a regulator of cell function J. Cell Biol.2012, 197, 351. [CrossRef]
- Sun Z., Guo S. S., Fässler R. Integrin-mediated mechanotransduction. J. Cell Biol. 215, 445–456 (2016). [CrossRef]
- Majhy B., Priyadarshinia P., Sen A. K. Effect of surface energy and roughness on cell adhesion and growth – facile surface modification for enhanced cell culture RSC Adv., 2021, 11, 15467. [CrossRef]
- Horbett T.A. The role of adsorbed proteins in animal cell adhesion Colloids and Surfaces B: Biointerfaces Vol 2, Issues 1–3, 14 March 1994, Pages 225-240. [CrossRef]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature 474: 179-183, 2011. [CrossRef]
- Meng, Z., Moroishi, T. & Guan, K. Mechanisms of Hippo pathway regulation. Genes & Development 30, 1-17 (2016). [CrossRef]
- Zinatizadeh, M. et al. The Hippo Tumor Suppressor Pathway (YAP/TAZ/TEAD/MST/LATS) and EGFR-RAS-RAF-MEK in cancer metastasis. Genes & Diseases (2019). [CrossRef]
- Pobbati, A. & Hong, W. A combat with the YAP/TAZ-TEAD oncoproteins for cancer therapy. Theranostics 10, 3622-3635 (2020). [CrossRef]
- Janmey P. A., Wells R. G., Assoian R. K., McCulloch C. A., From tissue mechanics to transcription factors. Differentiation 86, 112–120 (2013). [CrossRef]
- Johnson R., Halder G. The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 (2014). [CrossRef]
- Wang K.C., Yeh Y.T., Nguyen P., Limqueco E., Lopez J., Thorossian S., Guan K.L., Li Y.S. J., Chien S., Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 113, 11525–11530 (2016). [CrossRef]
- Li Y, Wang J, Zhong W (2021) Regulation and mechanism of YAP/TAZ in the mechanical microenvironment of stem cells Mol Med Rep. 2021 Jul; 24(1): 506. Published online 2021. [CrossRef]
- Samsonraj R. M., M.Raghunath,V. Nurcombe, J. H. Hui, A. J. van Wijnen, S.M. Cool, Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine, Stem Cells Translational Medicine, Volume 6, Issue 12, December 2017, 2173–2185. [CrossRef]
- Dupont S. et al., Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). [CrossRef]
- Dupont S., Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 343, 42–53 (2016). [CrossRef]
- Bao M., Xie J., Huck W. T. S. Recent Advances in Engineering the Stem Cell Microniche in 3D Adv. Sci. 2018, 5, 1800448. [CrossRef]
- Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12):771-785. [CrossRef]
- Heino J. Cellular signaling by collagen-binding integrins.Adv Exp Med Biol 2014;819:143-55. [CrossRef]
- Zeltz C, Gullberg D.J, The integrin-collagen connection - a glue for tissue repair? Cell Sci. 2016 Mar 15;129(6):1284. [CrossRef]
- Myllyharju, J. Intracellular Post-Translational Modifications of Collagens. In: Brinckmann, J., Notbohm H, Müller, P K (eds) Collagen. Topics in Current Chemistry, vol 247. Springer, Berlin, Heidelberg. [CrossRef]
- Kennett EC, Chuang CY, Degendorfer G, Whitelock JM, Davies MJ. Mechanisms and consequences of oxidative damage to extracellular matrix. Biochem Soc Trans. 2011, 39(5):1279-87. [CrossRef]
- Boin, F., Erre, G.L., Posadino, A.M. et al. Oxidative stress-dependent activation of collagen synthesis is induced in human pulmonary smooth muscle cells by sera from patients with scleroderma-associated pulmonary hypertension. Orphanet J Rare Dis 9, 123 (2014). [CrossRef]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12):a005058. Published 2011 Dec 1. [CrossRef]
- Komsa-Penkova R, Stavreva G, Belemezova K, Kyurkchiev S, Todinova S, Altankov G. Mesenchymal Stem-Cell Remodeling of Adsorbed Type-I Collagen-The Effect of Collagen Oxidation. Int J Mol Sci. 2022 Mar 11;23(6):3058. [CrossRef]
- Bao M., Xie J., Huck W. T. S. Recent Advances in Engineering the Stem Cell Microniche in 3D Adv. Sci. 2018, 5, 1800448. [CrossRef]
- Komsa-Penkova, R.; Koynova, R.; Kostov, G.; Tenchov, B., Discrete reduction of type I collagen thermal stability upon oxidation. Biophys. Chem. 2000, 83 (3), 185-195. [CrossRef]
- Horzum U, Ozdil B, Pesen-Okvur D. Step-by-step quantitative analysis of focal adhesions. MethodsX. 2014 Jul 7;1:56-9. [CrossRef]
- Saalfeld S. 2009. CLAHE (Contrast Limited Adaptive Histogram Equalization) Available from: http://rsbweb.nih.gov/ij/plugins/clahe/index.html (updated 2009/11/17) [Google Scholar].
- Sage D., Neumann F.R., Hediger F., Gasser S.M., Unser M. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans. Image Process. 2005;14(September (9)):1372–1383. PubMed PMID: 16190472 (Epub 2005/09/30) [PubMed] [Google Scholar]. [CrossRef]
- Park J., Kim D., Levchenko A., Allan C., Ker A., Smith C.L.C.,Tsimbouri P.M., Borsoi J., Neill S.O, Gadegaard N., Wolfenson H., Yang B., Sheetz M.P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol.2019, 81, 585. [CrossRef]
- Wang JH, Thampatty BP, Lin JS, Im HJ. Mechanoregulation of gene expression in fibroblasts. Gene. 2007 Apr 15;391(1-2):1-15. doi: 10.1016/j.gene.2007.01.014. Epub 2007 Jan 31. [CrossRef]
- Wolfenson H. Yang B., Sheetz M. P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol.2019, 81, 585. [CrossRef]
- Elango J, C. Hou, B. Bao, S.Wang, J. E.Maté Sánchez de Val and W. Wenhui (2022) The Molecular Interaction of Collagen with Cell Receptors for Biological Function, Polymers 14(5), 876; [CrossRef]
- Ruggiero F, Champliaud M.F., Garrone R, Aumailley M. Interactions between Cells and Collagen V Molecules or Single Chains Involve Distinct Mechanisms Experimental Cell Research,Volume 210, Issue 2, February 1994, Pages 215-223(1994). [CrossRef]
- Burridge K, Feramisco JR. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin”. Cell. 1980, 19 (3): 587–95. [CrossRef]
- Elango J, Hou C, Bao B, Wang S, Eduardo Maté Sánchez de Val J, Wenhui W. The Molecular Interaction of Collagen with Cell Receptors for Biological Function, Polymers 14(5), 876; (2022). [CrossRef]
- Park J., Kim D., Levchenko A. , Allan C., Ker A. , Smith C.L.C., Tsimbouri P.M. , Borsoi J., Neill S.O., Gadegaard N., Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells, Small 2020, 16, 1905422. [CrossRef]
| Cellular Parameters | Col | Col-Oxi | p |
| Cell spreading Area (μm2) | 216.0 | 179.6 | p> 0.05 |
| Cell Shape Index (CSI) | 0.25 | 0.33 | p< 0.05 |
| Cell Aspect ratio (CAR) | 3.33 | 2.61 | p> 0.05 |
| Cell | Col | Col-Oxi | p |
| Number of FA | 317 | 143 | p <0.05 |
| Total FA area (μm2) | 1085 | 406 | p <0.05 |
| Mean area per FA (μm2) | 3.66 | 2.84 | p >0.05 |
| Parameters | Col | Col-Oxi | p |
| Nuclear TAZ (pixels) | 229.3 | 178.2 | p <0.05 |
| Cytosolic TAZ (pixels) | 1.3 | 12.4 | p <0.05 |
| Ratio TAZ Nuclei/TAZ Cytosol | 176.2 | 14.4 | p >0.05 |
| Nuclear Parameters | Col | Col-Oxi | p |
| Nuclear Area per cell (M2 | 17.01 | 21.2 | p< 0.05 |
| Nuclear Shape Index (NCI) | 0.81 | 0.86 | p> 0.05 |
| Nuclear Aspect ratio (NAR) | 1.68 | 1.34 | p> 0.05 |
| Samples | RRMS (nm) | Ea (MPa) |
| Collagen native | 27.95 ± 5.1 | 56.6 ± 8 |
| Col-Oxidized | 5.51 ± 0.8 | 66.8 ± 5 |
| Col denatured | 1.04 ± 0.6 | 3610 ± 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





