Submitted:
07 January 2023
Posted:
10 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
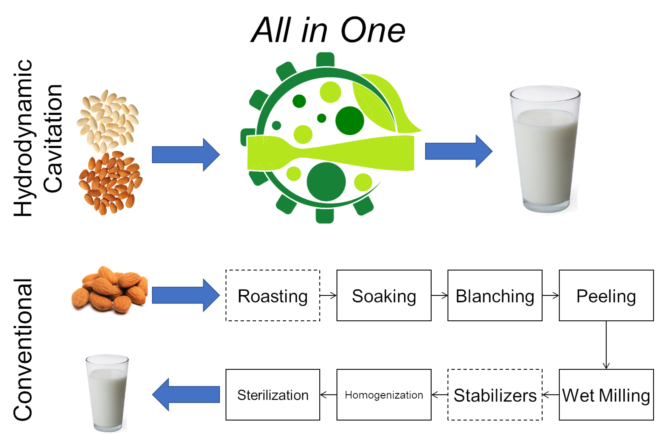
2. Technological Overview
- Roasting (optional, to increase the emulsion stability and the solubility of proteins): 95-100°, 30 min;
- Soaking in water: 4°C , 6 h;
- Blanching with peeling: in water 90°C, 3 min, in steam bath 85°C, 5-30 min.
- Wet milling: mass ratio 1:9 in water 18,000 rpm, 2 min;
- Filtration from solid residuals;
- Possible addition with stabilizers such as gums, sweeteners, salt, hydrocolloids, emulsifier, or fortified with micronutrients such calcium or some vitamins;
- Homogenization and sterilization (deactivation or extermination of spoilage or pathogenic microorganisms): ultra-high temperature (UHT) 140°C, few seconds, or ultra-high-pressure homogenization (UHPH) 350 MPa, 85 °C (with many variants).
3. Materials and Methods
3.1. Production of Aqueous Almond Extracts
3.2. Sampling and Microbiological, Nutritional, Total Polyphenols and Antiradical Activity Analyses
3.2.1. Sampling
3.2.2. Microbiological, nutritional and vitamin analyses
- Energy level: EU Regulation No. 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers (https://eur-lex.europa.eu/eli/reg/2011/1169/ojv);
- Total and unsaturated fat: Istisan report No. 1996/34 “Methods of analysis for the chemical control of foods”, pages 39 and 47, respectively (https://www.iss.it/en/rapporti-istisan);
- Total carbohydrates and sugar: Italian Ministerial Decree 03 February 1989 (https://www.gazzettaufficiale.it/eli/id/1989/07/20/089A3049/sg);
- Protein: Istisan report No. 1996/34 “Methods of analysis for the chemical control of foods”, page 17 (https://www.iss.it/en/rapporti-istisan);
- Fiber: Istisan report No. 1996/34 “Methods of analysis for the chemical control of foods”, page 73 (https://www.iss.it/en/rapporti-istisan);
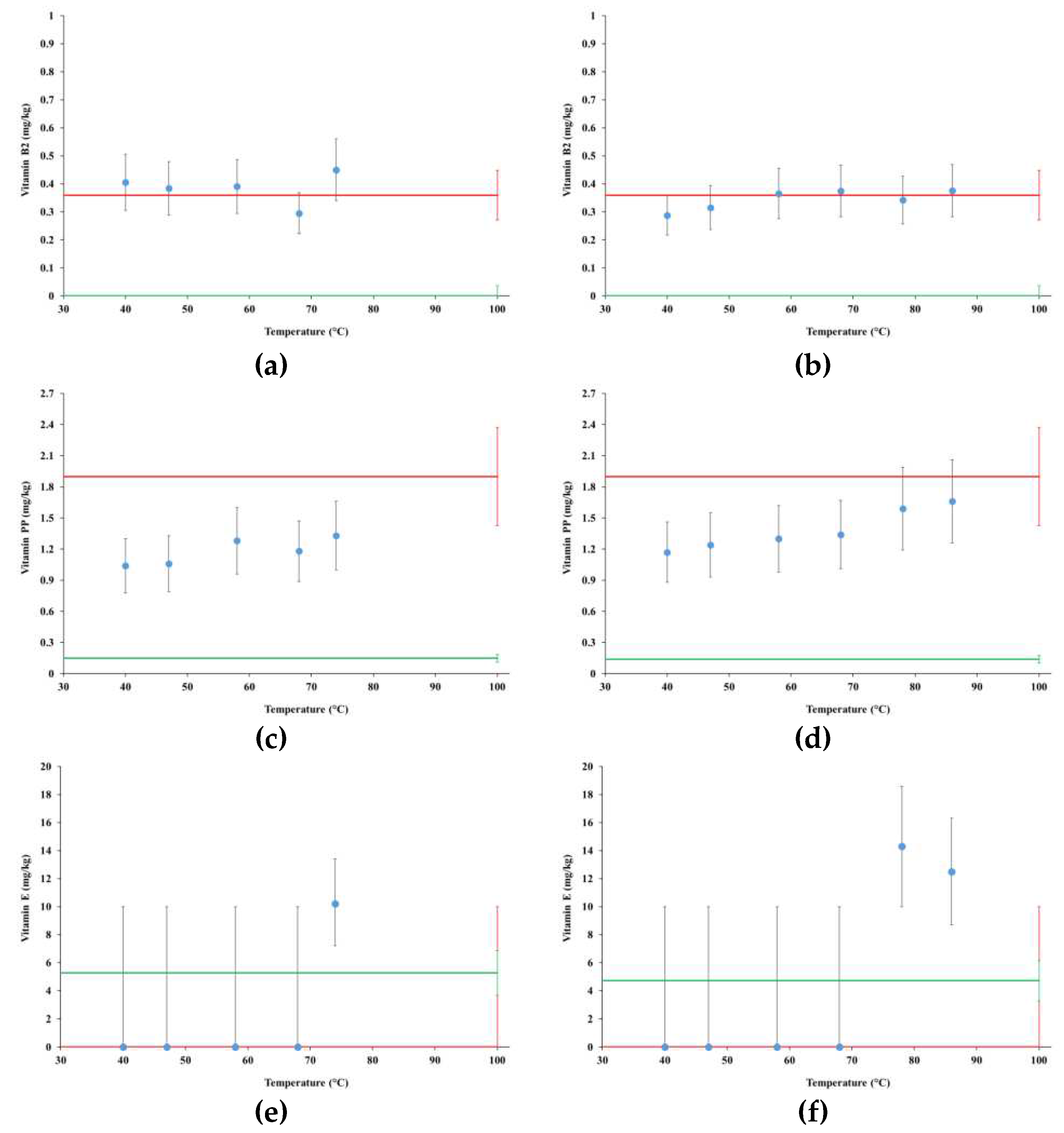
- Vitamin B2 and vitamin PP: AOAC 2015.14-2015 (http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=2990);
- Vitamin E: UNI EN 12822:2000 (https://store.uni.com/en/uni-en-12822-2000).
3.2.3. Total Polyphenols and Antiradical Activity
3.2.4. Potential levels
3.3. Mass balance
4. Results
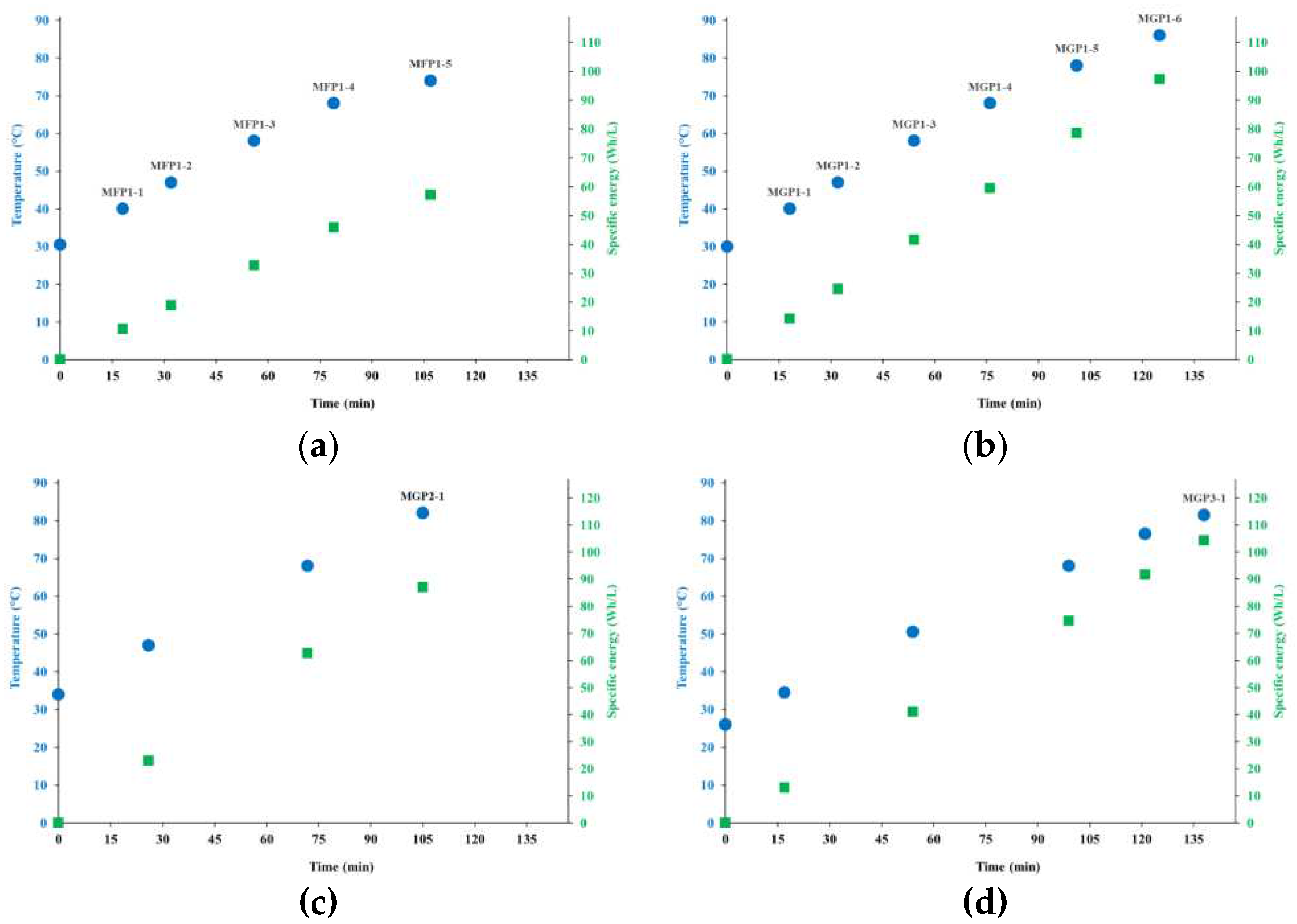
4.1. Processes and Energy Consumption
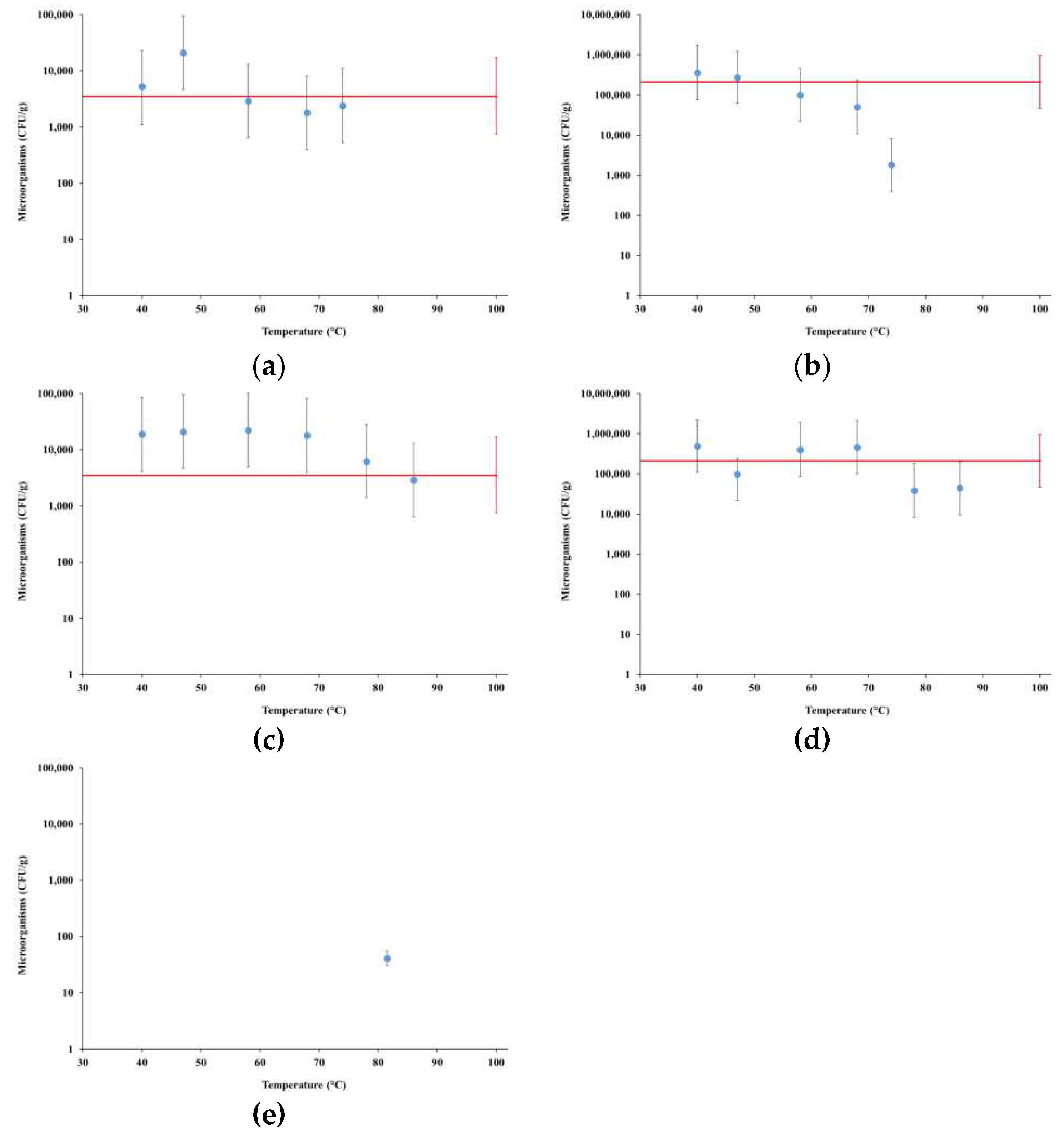
4.2. Microbiological Stability
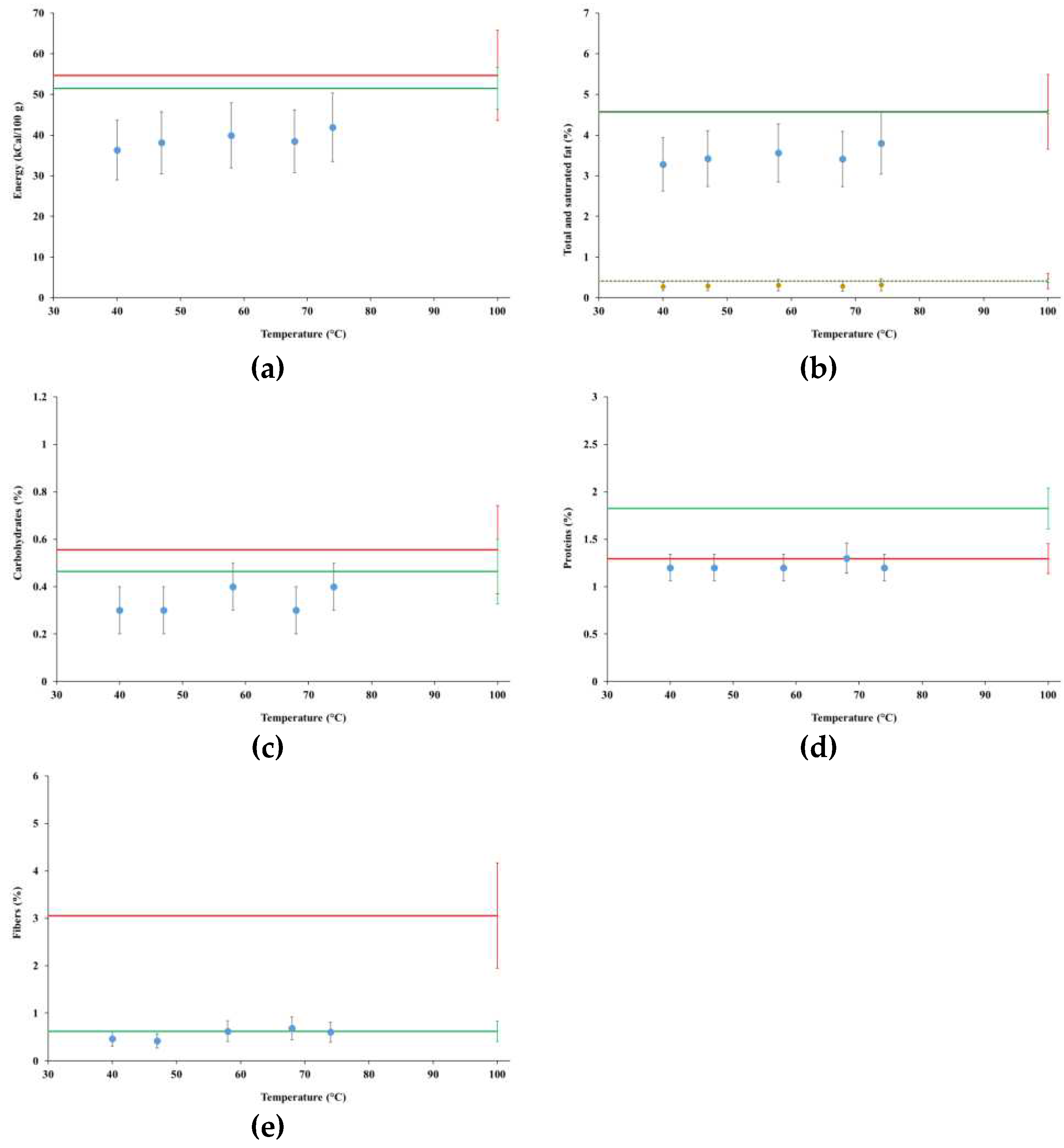
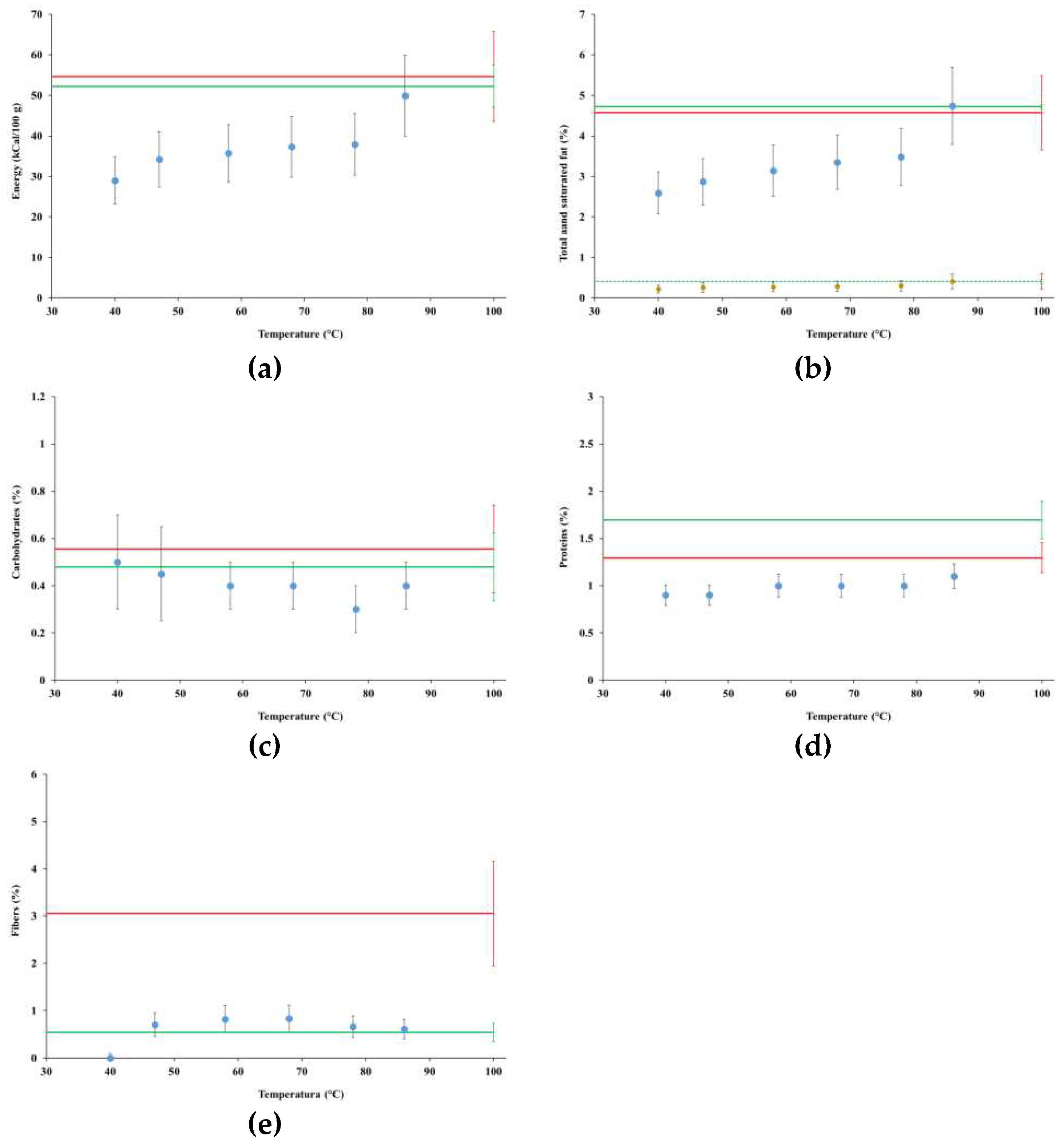
4.3. Nutritional Levels
4.3.1. Tests MFP1 and MGP1
4.3.2. Test MGP3
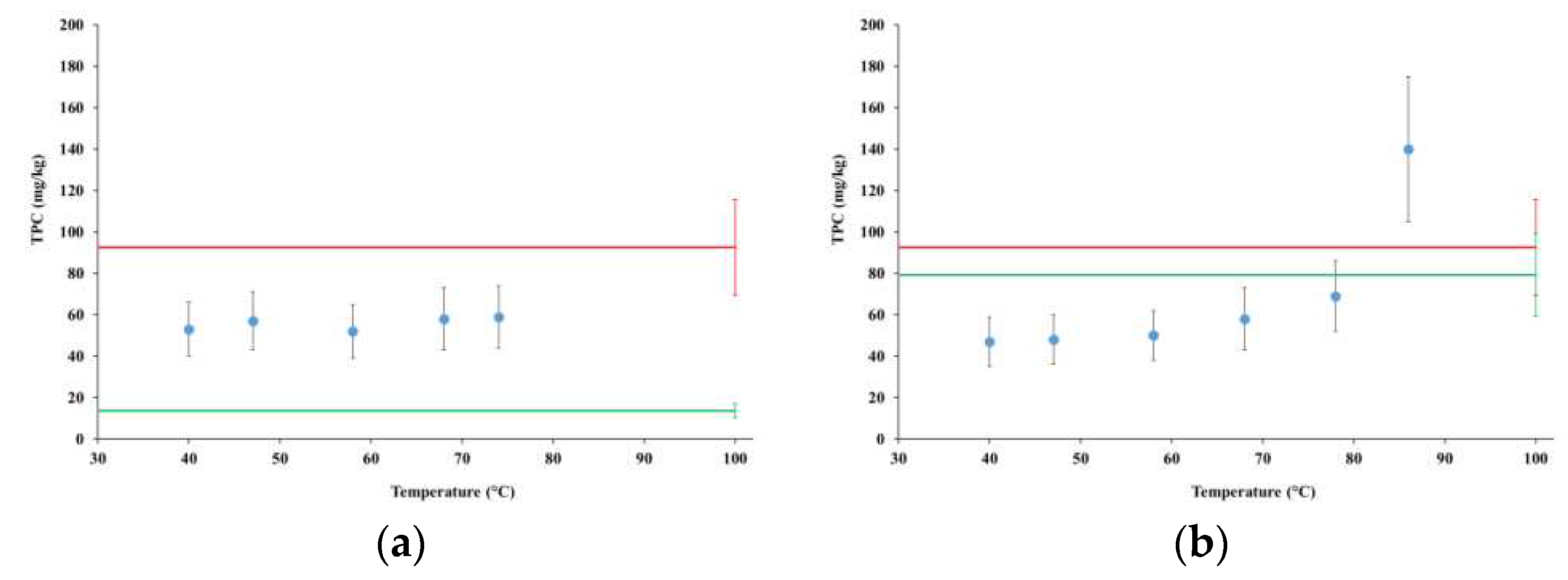
4.4. Total Polyphenol Content and Antiradical Actvity
4.4.1. TPC in tests MFP1 and MGP1
4.4.2. TPC and antiradical activity in tests MGP2 and MGP3
4.5. Vitamins
4.6. Mass balance for test MGP3
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bocker, R.; Silva, E.K. Innovative Technologies for Manufacturing Plant-Based Non-Dairy Alternative Milk and Their Impact on Nutritional, Sensory and Safety Aspects. Future Foods 2022, 5, 100098. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J Food Sci Technol 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, Present and Future: The Strength of Plant-Based Dairy Substitutes Based on Gluten-Free Raw Materials. Food Research International 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Valencia-Flores, D.C.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the Effects of Ultra-High-Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical, and Chemical Quality of Almond Beverages. J Food Sci 2013, 78. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Alami, M.; Mashkour, M.; Shahraki, M.H. Optimization of Ultrasound-Assisted Stabilization and Formulation of Almond Milk. J Food Process Preserv 2016, 40, 828–839. [Google Scholar] [CrossRef]
- Sanahuja, A.B.; Pérez, S.E.M.; Teruel, N.G.; García, A.V.; Moya, M.S.P. Variability of Chemical Profile in Almonds (Prunus Dulcis) of Different Cultivars and Origins. Foods 2021, 10, 153. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient Density and Nutritional Value of Milk and Plant-Based Milk Alternatives. International Dairy Journal 87 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Ozcan, M. A Review on Some Properties of Almond: Impact of Processing, Fatty Acids, Polyphenols, Nutrients, Bioactive Properties, and Health Aspects. J Food Sci Technol 2022. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Sanches Silva, A.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Josse, A.R.; Salvatore, S.; Brighenti, F.; Augustin, L.S.A.; Ellis, P.R.; Vidgen, E.; Rao, A.V. Almonds Decrease Postprandial Glycemia, Insulinemia, and Oxidative Damage in Healthy Individuals. J. Nutr 2006, 136, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hwang, H.J.; Kim, H.S.; Park, H. Time and Intervention Effects of Daily Almond Intake on the Changes of Lipid Profile and Body Composition Among Free-Living Healthy Adults. J Med Food 2018, 21, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J.; Novotny@ars, J.U.; Gov, J.A.N.; Baer@ars, D.U.; Gov, D.J.B. Almond Consumption and Processing Affects the Composition of the Gastrointestinal Microbiota of Healthy Adult Men and Women: A Randomized Controlled Trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.O.; Holbrook, M.; Duess, M.-A.; Dohadwala, M.M.; Hamburg, N.M.; Asztalos, B.F.; Milbury, P.E.; Blumberg, J.B.; Vita, J.A.; Mayer, J. Effect of Almond Consumption on Vascular Function in Patients with Coronary Artery Disease: A Randomized, Controlled, Cross-over Trial. Nutr J 2015, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, ; Augustine; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, R.; E.; Lapsley, K.G.; et al. Dose Response of Almonds on Coronary Heart Disease Risk Factors: Blood Lipids, Oxidized Low-Density Lipoproteins, Lipoprotein(a), Homocysteine, and Pulmonary Nitric Oxide A Randomized, Controlled, Crossover Trial. Circulation 2002. [CrossRef]
- Li, S.C.; Liu, Y.H.; Liu, J.F.; Chang, W.H.; Chen, C.M.; Chen, C.Y.O. Almond Consumption Improved Glycemic Control and Lipid Profiles in Patients with Type 2 Diabetes Mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus Dulcis L.) Skin. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial Potential of Polyphenols Extracted from Almond Skins. Lett Appl Microbiol 2010, 51, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.O.; Blumberg, J.B. In Vitro Activity of Almond Skin Polyphenols for Scavenging Free Radicals and Inducing Quinone Reductase. J Agric Food Chem 2008, 56, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-Based Milk Substitutes: Bioactive Compounds, Conventional and Novel Processes, Bioavailability Studies, and Health Effects. J Funct Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Zabini, F. Hydrodynamic Cavitation in Beer and Other Beverage Processing. In Reference Module in Food Science; Elsevier, 2020; pp. 369–394.
- Meneguzzo, F.; Zabini, F.; Albanese, L.; Crisci, A. Novel Affordable, Reliable and Efficient Technologies to Help Addressing the Water-Energy-Food Nexus. European Journal of Sustainable Development 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Zabini, F. Agri-Food and Forestry Sectors for Sustainable Development; Sustainable Development Goals Series; Springer International Publishing: Cham, 2021; ISBN 978-3-030-66283-7. [Google Scholar]
- Gallina, L.; Cravotto, C.; Capaldi, G.; Grillo, G.; Cravotto, G. Plant Extraction in Water: Towards Highly Efficient Industrial Applications. Processes 2022, 10, 2233. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Beer-Brewing Powered by Controlled Hydrodynamic Cavitation: Theory and Real-Scale Experiments. J Clean Prod 2017, 142, 1457–1470. [Google Scholar] [CrossRef]
- Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies Alba Mill.) Needles. Foods 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L.B. dos S.; Carlo, A. de; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.J.; Fryer, P.J.; Zuidam, N.J. Intensification of Protein Extraction from Soybean Processing Materials Using Hydrodynamic Cavitation. Innovative Food Science and Emerging Technologies 2017, 41, 47–55. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-Based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr Rev Food Sci Food Saf 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.C.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Reviews International 2021. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr Rev Food Sci Food Saf 2017, 16. [Google Scholar] [CrossRef]
- Tabib, M.; Tao, Y.; Ginies, C.; Bornard, I.; Rakotomanomana, N.; Remmal, A.; Chemat, F. A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and Its Effects on Structure, Polyphenols, Lipids, and Proteins Quality. Applied Sciences 2020, 10, 3628. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of Different Processing Treatments on Almond (Prunus Dulcis) Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Applied Sciences 2019, 9, 766. [Google Scholar] [CrossRef]
- Rojas, M.L.; Kubo, M.T.K.; Miano, A.C.; Augusto, P.E.D. Ultrasound Processing to Enhance the Functionality of Plant-Based Beverages and Proteins. Curr Opin Food Sci 2022, 48, 100939. [Google Scholar] [CrossRef]
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled Hydrodynamic Cavitation: A Review of Recent Advances and Perspectives for Greener Processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Zhu, J. Recent Developments in Hydrodynamic Cavitation Reactors : Cavitation Mechanism, Reactor Design, and Applications. Engineering 2022. [Google Scholar] [CrossRef]
- Salve, A.R.; Pegu, K.; Arya, S.S. Comparative Assessment of High-Intensity Ultrasound and Hydrodynamic Cavitation Processing on Physico-Chemical Properties and Microbial Inactivation of Peanut Milk. Ultrason Sonochem 2019. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J Food Sci Technol 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Iorio, M.C.; Bevilacqua, A.; Corbo, M.R.; Campaniello, D.; Sinigaglia, M.; Altieri, C. A Case Study on the Use of Ultrasound for the Inhibition of Escherichia Coli O157:H7 and Listeria Monocytogenes in Almond Milk. Ultrason Sonochem 2019, 52, 477–483. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Alami, M.; Mashkour, M.; Shahraki, M.H. Optimization of Ultrasound-Assisted Stabilization and Formulation of Almond Milk. J Food Process Preserv 2016, 40, 828–839. [Google Scholar] [CrossRef]
- Dhakal, S.; Giusti, M.M.; Balasubramaniam, V.M. Effect of High Pressure Processing on Dispersive and Aggregative Properties of Almond Milk. J Sci Food Agric 2016, 96, 3821–3830. [Google Scholar] [CrossRef]
- Briviba, K.; Gräf, V.; Walz, E.; Guamis, B.; Butz, P. Ultra High Pressure Homogenization of Almond Milk: Physico-Chemical and Physiological Effects. Food Chem 2016, 192, 82–89. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Siddique, R.; Hussain, A.; Ahmad, N.; Rehman, A.; Siddeeg, A.; Alfarga, A.; Alshammari, G.M.; Yahya, M.A. Thermosonication Effect on Bioactive Compounds, Enzymes Activity, Particle Size, Microbial Load, and Sensory Properties of Almond (Prunus Dulcis) Milk. Ultrason Sonochem 2021, 78, 105705. [Google Scholar] [CrossRef]
- Valencia-Flores, D.C.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the Effects of Ultra-High-Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical, and Chemical Quality of Almond Beverages. J Food Sci 2013, 78, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Ferragut, V.; Hernández-Herrero, M.; Veciana-Nogués, M.T.; Borras-Suarez, M.; González-Linares, J.; Vidal-Carou, M.C.; Guamis, B. Ultra-High-Pressure Homogenization (UHPH) System for Producing High-Quality Vegetable-Based Beverages: Physicochemical, Microbiological, Nutritional and Toxicological Characteristics. J Sci Food Agric 2015, 95, 953–961. [Google Scholar] [CrossRef]
- Pica, A.L.; Silvestri, C.; Cristofori, V. Cultivar-Specific Assessments of Almond Nutritional Status through Foliar Analysis. Horticulturae 2022, 8, 822. [Google Scholar] [CrossRef]
- Piscopo, A.; Romeo, F. v.; Petrovicova, B.; Poiana, M. Effect of the Harvest Time on Kernel Quality of Several Almond Varieties (Prunus Dulcis (Mill.) D.A. Webb). Sci Hortic 2010, 125, 41–46. [Google Scholar] [CrossRef]
- Montalbano, L.M.; Solano, M.; Vaccaro, P. Vegetable Beverage Based on Almonds Cream Ready to Use 2006, 3.
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. Journal of Biological Chemistry 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; Heimler, D. HPLC-DAD/MS Characterization of Flavonoids and Hydroxycinnamic Derivatives in Turnip Tops (Brassica Rapa L. Subsp. Sylvestris L.). J Agric Food Chem 2006, 54, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, A.; Chen, Y. Degradation Kinetics of Total Anthocyanins and Formation of Polymeric Color in Blueberry Hydrothermodynamic (HTD) Processing. J Food Eng 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Bhukya, J.; Mohapatra, D.; Naik, R. Hydrodynamic Cavitation Processing of Ascorbic Acid Treated Precooled Sugarcane Juice for Physiochemical, Bioactive, Enzyme Stability, and Microbial Safety. J Food Process Eng 2022, e14209. [Google Scholar] [CrossRef]
- Devnani, B.; Ong, L.; Kentish, S.; Gras, S. Heat Induced Denaturation, Aggregation and Gelation of Almond Proteins in Skim and Full Fat Almond Milk. Food Chem 2020, 325, 126901. [Google Scholar] [CrossRef]
- Kamal, H.; Ali, A.; Manickam, S.; Le, C.F. Impact of Cavitation on the Structure and Functional Quality of Extracted Protein from Food Sources - An Overview. Food Chem 2023, 407, 135071. [Google Scholar] [CrossRef]
- Domínguez Avila, J.A.; Wall Medrano, A.; Ruiz Pardo, C.A.; Montalvo González, E.; González Aguilar, G.A. Use of Nonthermal Technologies in the Production of Functional Beverages from Vegetable Ingredients to Preserve Heat-Labile Phytochemicals. J Food Process Preserv 2018, 42, e13506. [Google Scholar] [CrossRef]
- Özcan, M.M. A Review on Some Properties of Almond: Impact of Processing, Fatty Acids, Polyphenols, Nutrients, Bioactive Properties, and Health Aspects. J Food Sci Technol 2022. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus Dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenols. J Agric Food Chem 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial Potential of Polyphenols Extracted from Almond Skins. Lett Appl Microbiol 2010, 51, 83–89. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus Dulcis L.) Skin. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci Technol 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Alami, M.; Mashkour, M.; Shahraki, M.H. Optimization of Ultrasound-Assisted Stabilization and Formulation of Almond Milk. J Food Process Preserv 2016, 40, 828–839. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Dhakal, S.; Liu, C.; Zhang, Y.; Roux, K.H.; Sathe, S.K.; Balasubramaniam, V.M. Effect of High Pressure Processing on the Immunoreactivity of Almond Milk. Food Research International 2014, 62, 215–222. [Google Scholar] [CrossRef]


| Technology | Application | Process | Main results | Reference |
|---|---|---|---|---|
| US | Almond beverage: disinfection | 130 W/ 80%/20 kHz 8 min/6 s of pulse |
Escherichia coli (O157:H7): 5.12 to 3.81 log CFU/mL. Listeria monocytogenes: reduction by 1 log CFU/mL. |
[40] |
| US | Almond beverage: physicochemical | 300W/20kHz/100%0 to 5 minutes | Higher Brix degree and physical stability. Decreased viscosity and suspended particles size. |
[41] |
| HHP | Almond beverage: physicochemical | HHP (450 and 600 MPa for 0, 30, 60, 180, 300, and 600 s at 30 °C) Control: Traditional thermal process (0, 30, 180, and 300 s at 72, 85, and 99 °C). |
Aggregation and coagulation of almond proteins. Improved sensorial properties. |
[42] |
| HPP | Almond beverage: physicochemical | 350 MPa and 85°C for 15 seconds. | Microbiological stability. Increase of particle size. No change of cytotoxic, genotoxic, and antigenotoxic activity. | [43] |
| TS | Almond beverage: disinfection, physicochemical, micronutrients | TS: 600 W/40 kHz/30, 45, and 60 °C for 10, 20, 30, and 40 min Control: pasteurization (60 s at 90 °C) |
Particle size reduction due to acoustic cavitation. Improvement of rheological properties. Increased bioavailability of phenolics. |
[44] |
| UHPH | Almond beverage: disinfection, chemical-physical improvement | 200 and 300 MPa at 55, 65, and 75 °C, with emulsifying agent (lecithin) | 200 MPa with 55°C inlet temperature improves over conventional pasteurization. | [45] |
| UHPH | Almond and soy beverages: physicochemical, microbiological, nutritional and toxicological | 200 MPa, 55°C; 300 MPa, 75 °C. Control: UHT. |
300 MPa, 75 °C led to a complete inactivation of microorganisms, improved colloidal stability | [46] |
| Test ID |
Almond material |
Mass of almonds(kg) |
Water Volume (L) |
Concentration (%) |
ProcessTime (min) |
Process temperatures(°C) |
|---|---|---|---|---|---|---|
| MFP1 | Peeled, flour | 15 | 187.5 | 7.4 | 107 | 30-74 |
| MGP1 | Peeled, fine grain | 12 | 150 | 7.4 | 125 | 30-86 |
| MGP2 | Peeled, fine grain | 56 | 150 | 27.2 | 105 | 34-82 |
| MGP3 | Whole, coarse grain | 33 | 150 | 18.0 | 138 | 26-82 |
| Test ID | Sample type 1 | Microbiological 2 | Nutritional | Total polyphenols |
Antiradical activity | |
|---|---|---|---|---|---|---|
| T0 | Shelf life | |||||
| MFP1 | Raw | X | X | X | X | |
| MFP1 | Extract | X | X | X | ||
| MGP1 | Raw | X | X | X | X | |
| MGP1 | Extract | X | X | X | ||
| MGP2 | Extract | X | X | |||
| MGP3 | Raw | X | X | X | ||
| MGP3 | Extract | X | X | X | X | |
| Commercial | Extract | X | X | X | X | |
| Quantity | Sample MGP3 | Potential level |
|---|---|---|
| Energy (kCal/100 g) | 52 | 119 |
| Total Fat (%) | 4.3±0.9 | 10.6±2.1 |
| Saturated fat (%) | 0.3±0.1 | 0.8±0.2 |
| Carbohydrates (%) | 0.8±0.2 | 1.8±0.4 |
| Proteins (%) | 2.5±0.5 | 4.3±0.9 |
| Fibers (%) | 0.8±0.2 | 3.9±0.8 |
| Test ID |
TPC in extract(mg/kg) | TPC potential level (mg/kg) |
TPC extraction yield (%) |
IC50 (μL/mL) |
|---|---|---|---|---|
| MGP2 | 193±20 | 370±93 | 52% | 42.93±0.09 |
| MGP3 | 141±14 | 174±17 | 81% | 28.13±0.73 |
| Material | Fresh mass (g) |
Average ratio to fresh mass(%) |
Dry Biomass (g) |
Average ratio to dry mass 1 (%) |
|---|---|---|---|---|
| Whole 2 | 47.3±1.6 | 100 | 8.73±0.42 | 18.5 |
| Pellet | 24.0±0.9 | 50.8 | 6.29±0.31 | 26.2 |
| Supernatant | 23.3±0.8 | 49.2 | 2.38±0.14 | 10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





