Submitted:
09 January 2023
Posted:
10 January 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Experimental
Materials and Methods
Materials Characterization
Results and Discussion
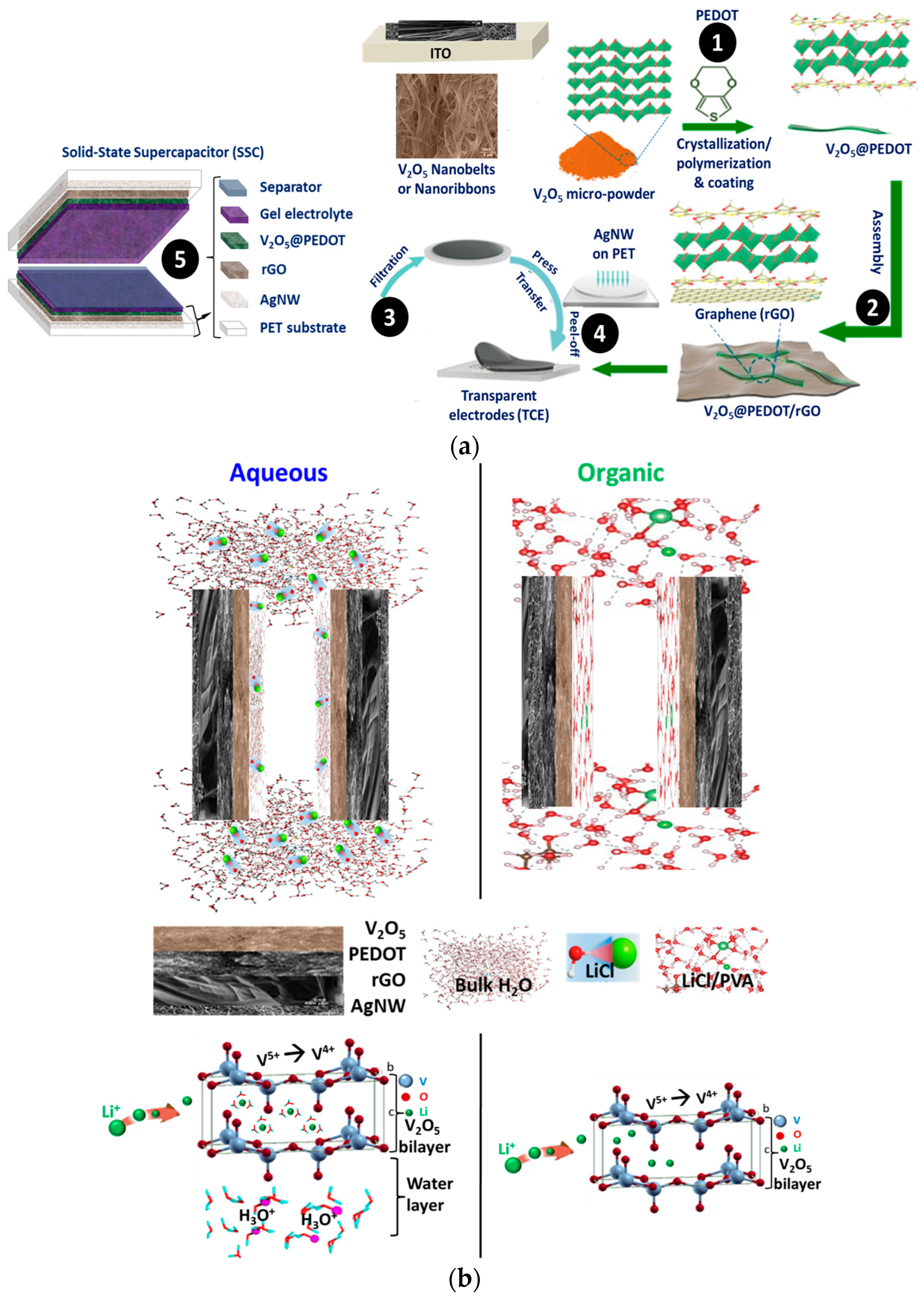
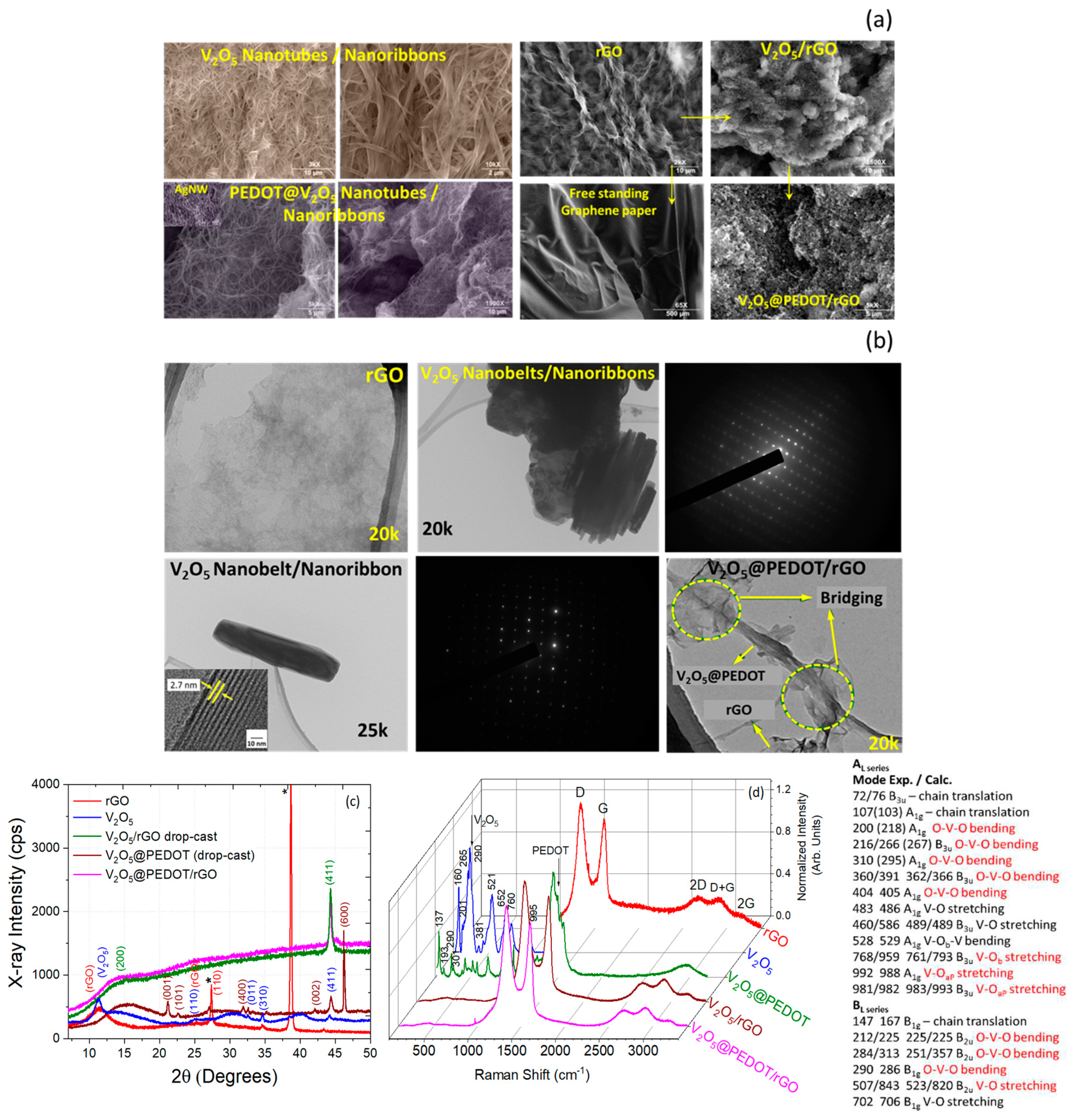
Microscopic Structure and Morphology
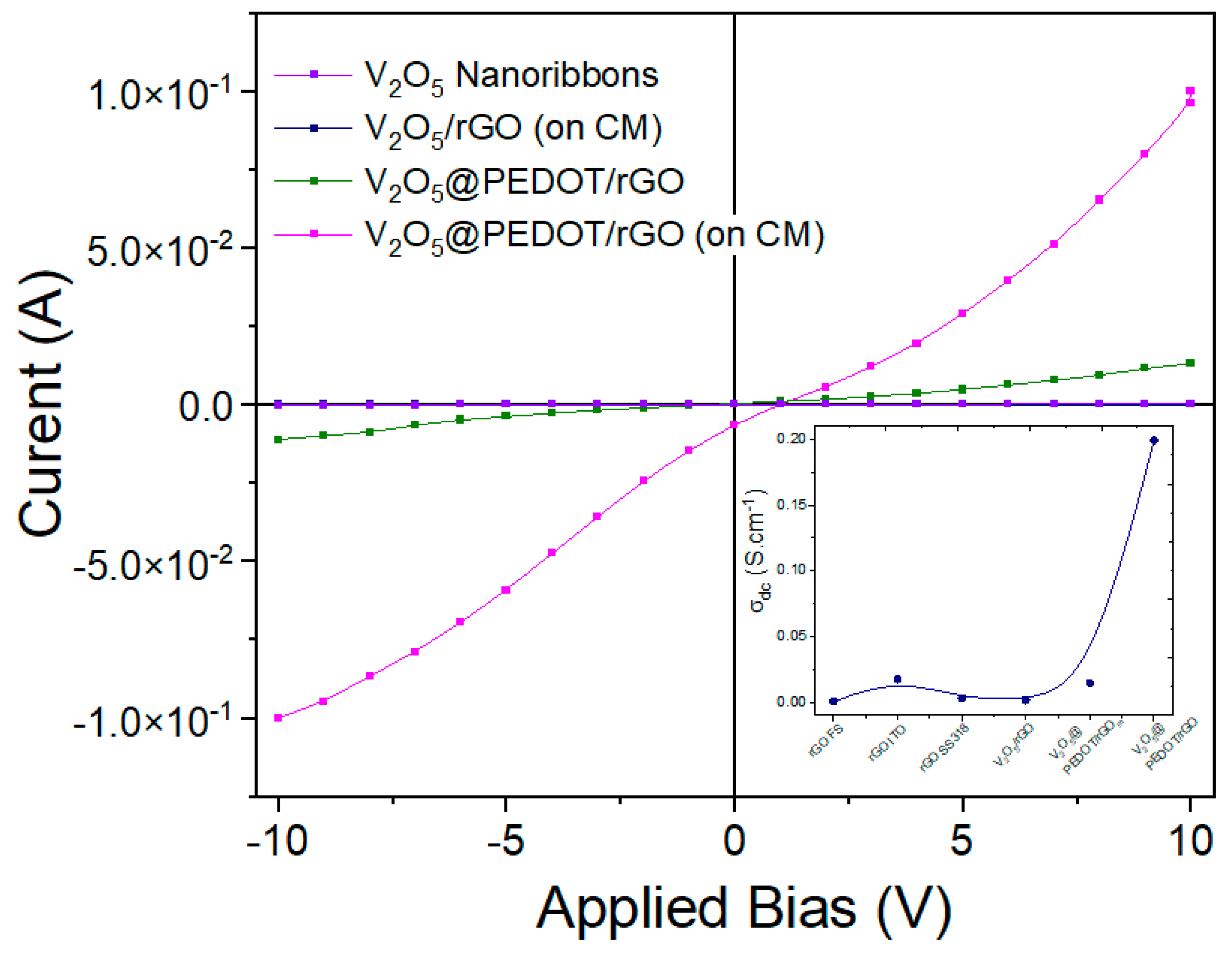
Transparency and Transferability of V2O5@PEDOT/rGO
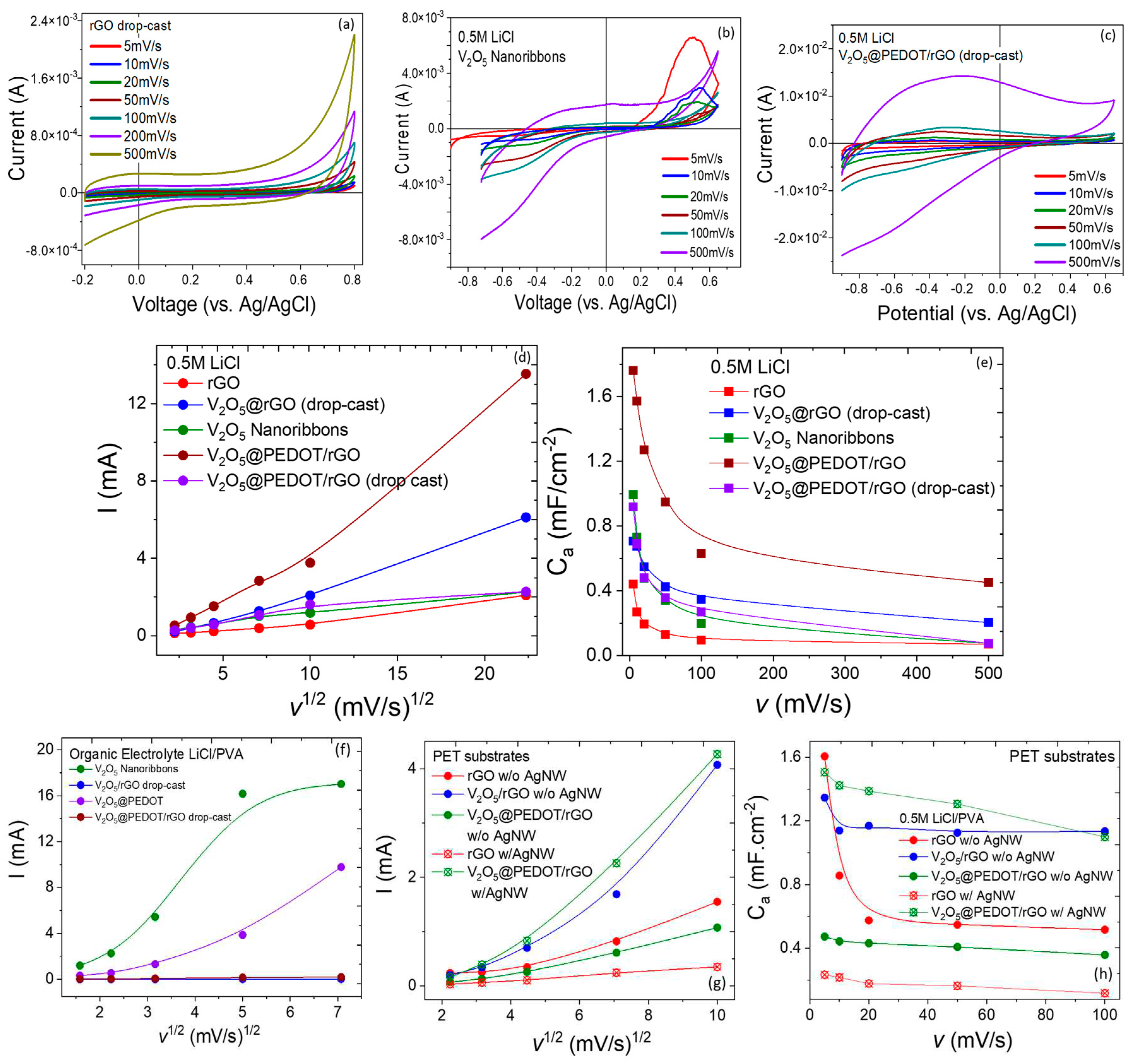
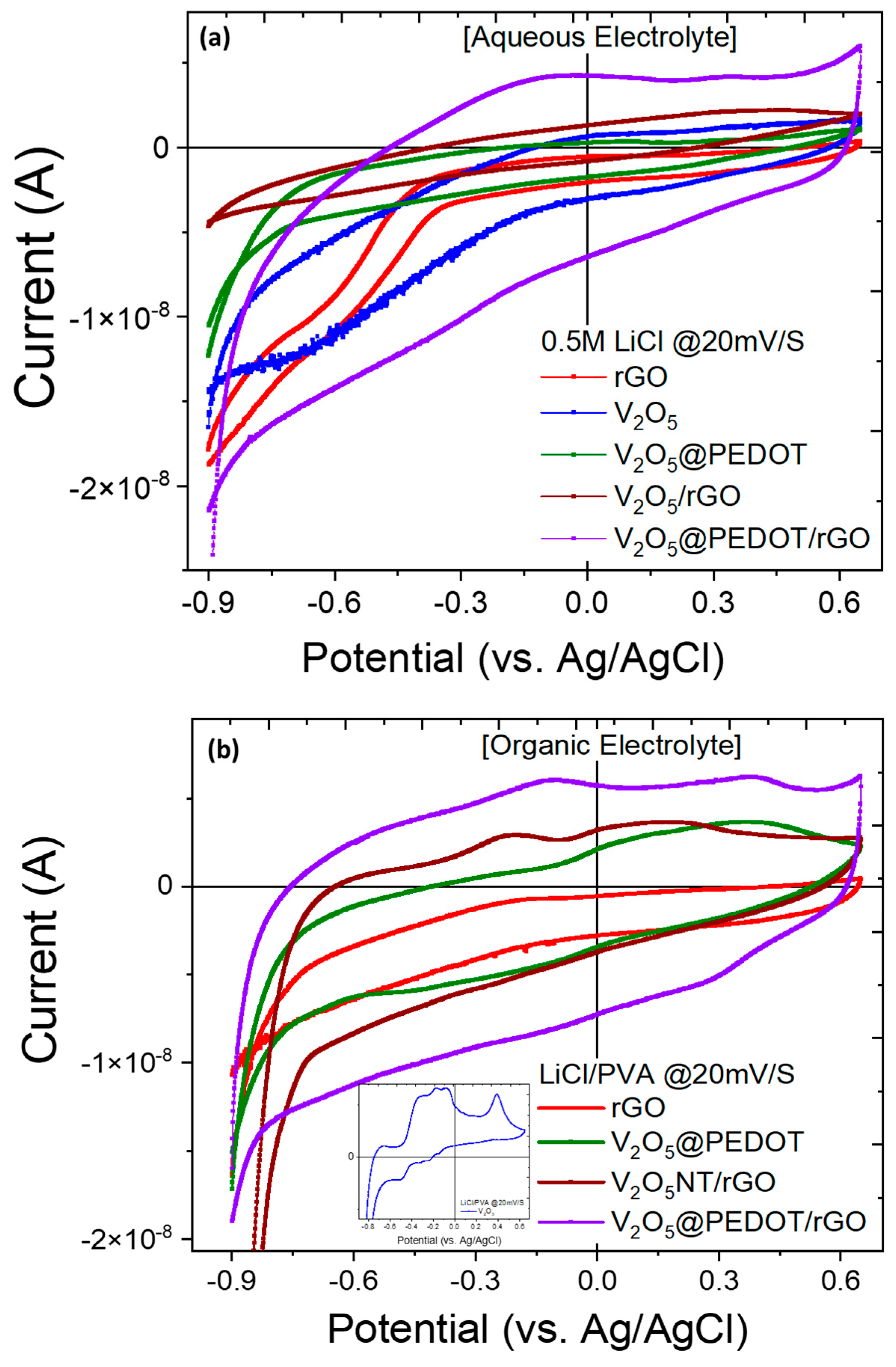
Electrochemical Properties
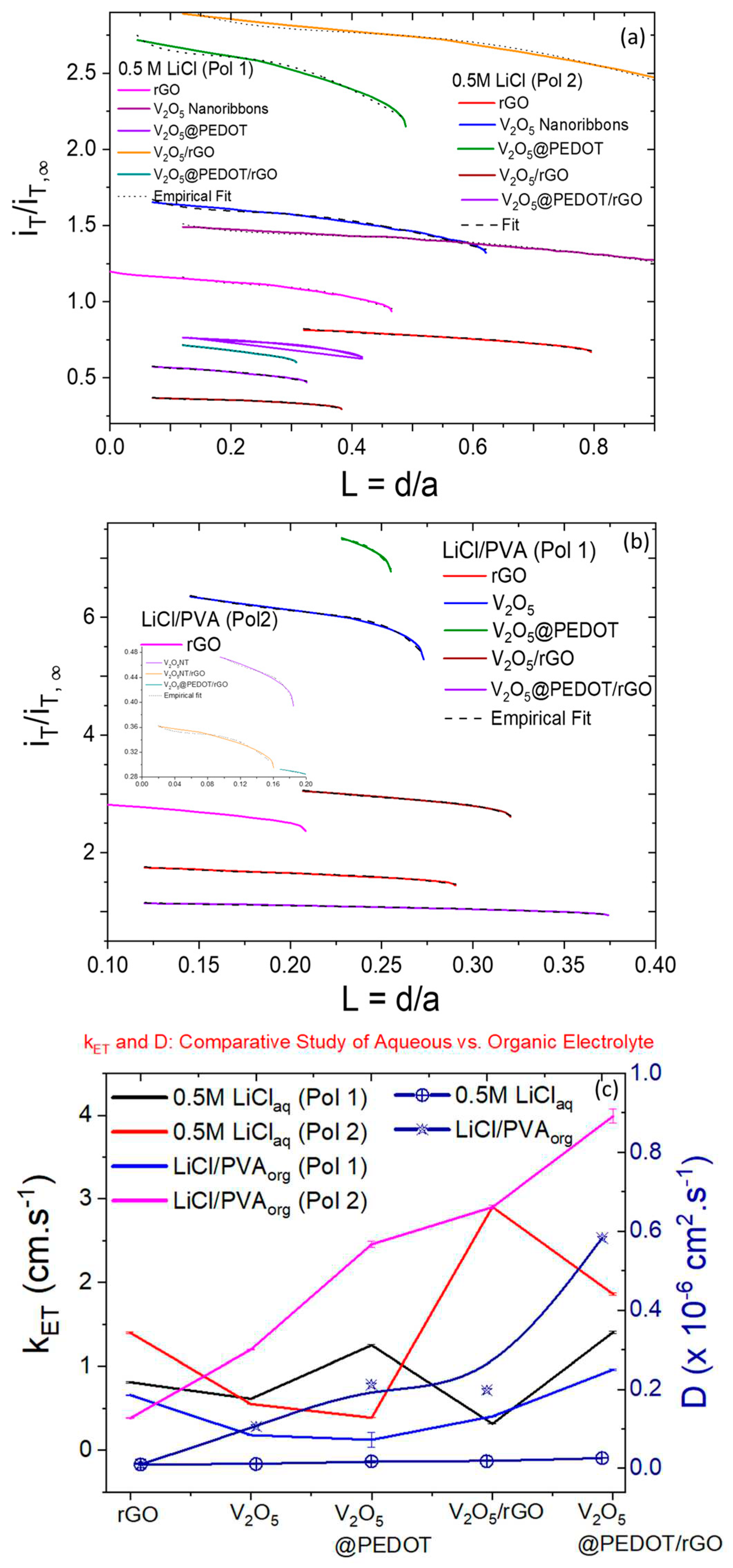
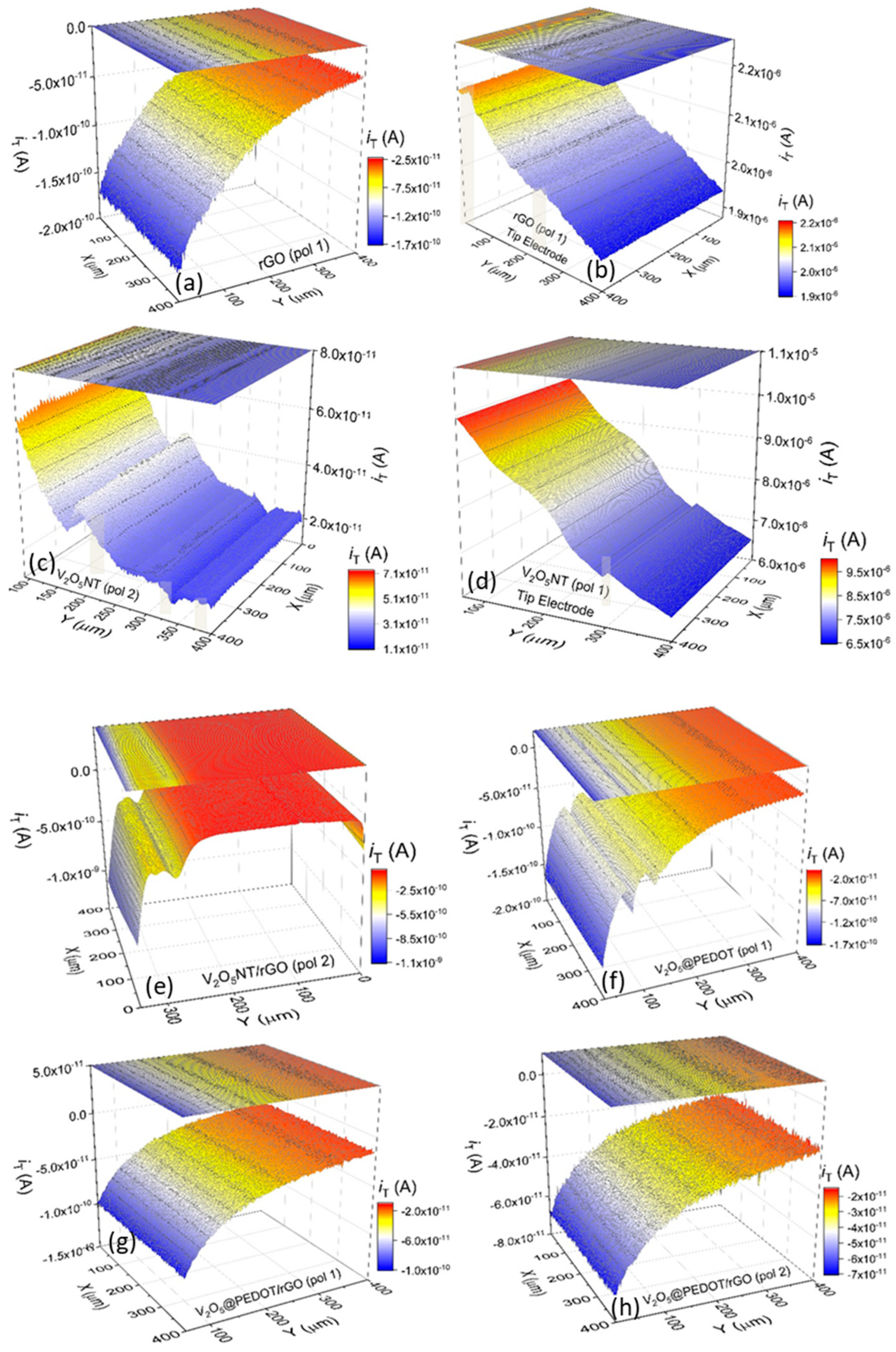
Electrochemical Imaging and Determination of Heterogeneous Electron Transfer Rate
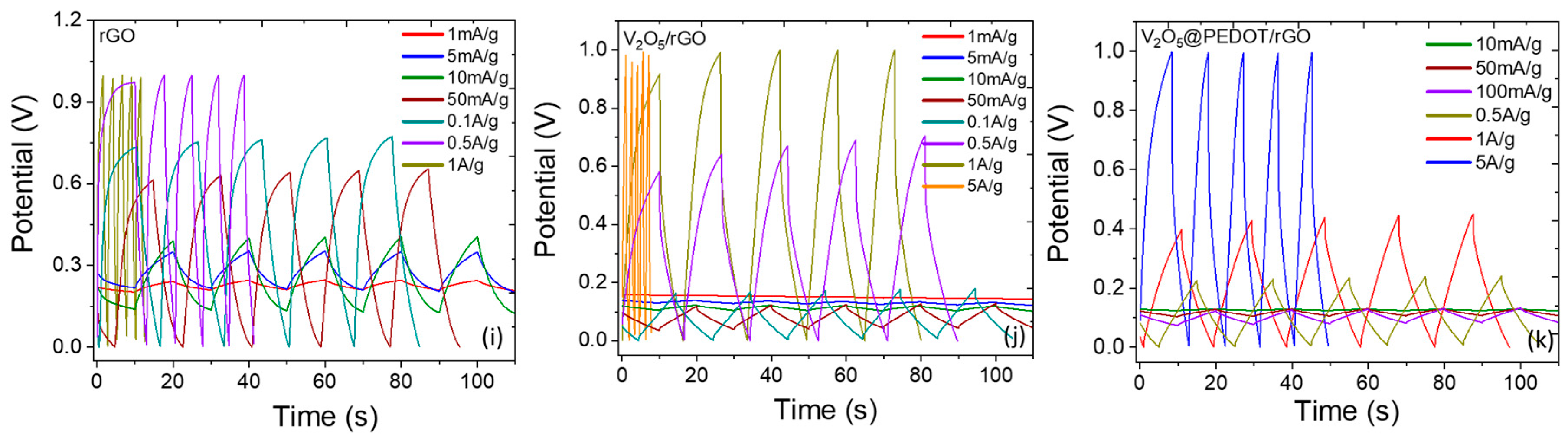
Electrochemical Performance of Transparent Symmetric Devices
Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- S. Chu, Y. Cui, N. Liu, The path towards sustainable energy. Nat. Mater. 16, 16-22 (2016). [CrossRef]
- M. Armand, Polymer solid electrolytes - an overview. Solid State Ionics 9(10), 745-754 (1983). [CrossRef]
- Y. Shim, H. J. Kim, Y. J. Jung, Graphene-based supercapacitors in the parallel-plate electrode configuration: Ionic liquids versus organic electrolytes. Faraday Discuss. 154, 249-263 (2012).
- K. Ellmer, Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photon. 6, 809-817 (2012). [CrossRef]
- R. G. Gordon, Criteria for choosing transparent conductors. MRS Bull. 25, 52-57 (2011). [CrossRef]
- C.-C. Kim, H.-H. Lee, K.H. Oh, J.-Y. Sun, Highly stretchable, transparent ionic touch panel. Science 353, 682-687 (2016). [CrossRef]
- T. M. Higgins, J.N. Coleman, Avoiding resistance limitations in high-performance transparent supercapacitor electrodes based on large-area, high-conductivity PEDOT: PSS films. ACS Appl. Mater. Interfaces 7, 16495-16506 (2015). [CrossRef]
- C. F. Zhang, T. M. Higgins, S.-H. Park, S. E. O’Brien, D. Long, J. N. Coleman, V. Nicolosi, Highly flexible and transparent solid-state supercapacitors based on RuO2/PEDOT: PSS conductive ultrathin films. Nano Energy 28, 495-505 (2016). [CrossRef]
- S. De, T. M. Higgins, P. E. Lyons, E. M. Doherty, P. N. Nirmalraj, W. J. Blau, J.J. Boland, J. N. Coleman, Silver nanowire networks as flexible, transparent, conducting films: extremely high DC to optical conductivity ratios. ACS Nano 3, 1767-1774 (2009). [CrossRef]
- Y.-H. Liu, J.-L. Xu, X. Gao, Y.-L. Sun, J.-J. Lv, S. Shen, L.-S. Chen, S.-D. Wang, Freestanding transparent metallic network based ultrathin, foldable, and designable supercapacitors. Energy Environ. Sci. 10, 2534-2543 (2017). [CrossRef]
- G. Li, Y. Liu, H. Zhou, Layered graphite foil/poly(3,4-ethylenedioxythiophene) electrode-enabled flexible electrochemical capacitors with observably boosted performances. J. Electroanal. Chem. 920, 116568-116574 (2022). [CrossRef]
- L. Hu, H.S. Kim, J.-Y. Lee, P. Peumans, Y. Cui, Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 4, 2955-2963 (2010). [CrossRef]
- B. W. Wang, S. Jiang, Q.-B. Zhu, Y. Sun, J. Luan, P.-X. Hou, S. Qiu, Q.-W. Li, C. Liu, D.-M. Sun, H.-M. Cheng, Continuous fabrication of meter-scale single-wall carbon nanotube films and their use in flexible and transparent integrated circuits. Adv. Mater. 30, e1802057 (2018). [CrossRef]
- S. Pang, Y. Hernandez, X. Feng, K. Müllen, Graphene as transparent electrode material for organic electronics. Adv. Mater. 23, 2779-2795 (2011). [CrossRef]
- S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, et. al., Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 5, 574-578 (2010). [CrossRef]
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, et. al., Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457, 706-710 (2009). [CrossRef]
- F. Chen, P. Wan, H. Xu, X. Sun, Flexible transparent supercapacitors based on hierarchical nanocomposite films. ACS Appl. Mater. Interfaces 9, 17865-17871 (2017). [CrossRef]
- S. Gupta, C. Price, Investigating graphene/conducting polymer hybrid layered composites as pseudocapacitors: Interplay of heterogeneous electron transfer, electric double layers and mechanical stability. Composites Part B 105, 46-59 (2016). [CrossRef]
- C. F. Zhang, V. Nicolosi, Graphene and MXene-based transparent conductive electrodes and supercapacitors. Energy Storage Mater. 16, 102-125 (2019). [CrossRef]
- S. A. Hasan, J. L. Rigueur, R. R. Harl, A. J. Krejci, I. G.-Juan, B. R. Rogers, J. H. Dickerson, Transferable graphene oxide films with tunable microstructures. ACS Nano 4, 7367-7372 (2010). [CrossRef]
- Y. Jiang, J. Liu, Definitions of pseudocapacitive materials: a brief review. Energy Environ. Mater. 2, 30-37 (2019). [CrossRef]
- B. Saravanakumar, K. K. Purushothaman, G. Muralidharan, High performance supercapacitor based on carbon coated V2O5 nanorods. J. Electroanal. Chem. 758, 111-116 (2015). [CrossRef]
- S. Gupta. S. B. Carrizosa, B. McDonald, J. Jasinski, N. Dimakis, Graphene-family nanomaterials assembled with cobalt oxides and cobalt nanoparticles as hybrid supercapacitive electrodes and enzymeless glucose detection platforms. J. Mater. Res. 32, 301-322 (2017). [CrossRef]
- S. Gupta, M. vanMeveren and J. Jasinski, Investigating Electrochemical Properties and Interfacial Processes of Manganese Oxides/Graphene Hybrids as High-Performance Supercapacitor Electrodes. Int. J. Electrochem. Sci. 10, 10272-10291 (2015). [CrossRef]
- S. Bai, K. Zhang, L. Wang, J. Sun, R. Luo, D. Li and A. Chen, Synthesis mechanism and gas-sensing application of nanosheet-assembled tungsten oxide microspheres. J. Mater. Chem. A 2, 7927-7934 (2014). [CrossRef]
- G. Wang L. Zhang, J. Zhang, A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 41, 797-828 (2012). [CrossRef]
- R. Raccichini, A. Varzi, S. Passerini and B. Scrosati, A. Varzi, S. Passerini, B. Scrosati, The role of graphene for electrochemical energy storage. Nat. Mater. 14, 271 (2015). [CrossRef]
- T. O. Wehling, K. S. Novoselov, S. V. Morozov, E. E. Vdovin, M. I. Katsnelson, A. K. Geim, A. I. Lichtenstein, Molecular doping in graphene. Nano Lett. 8, 173–177 (2008). [CrossRef]
- M. Lee, S. K. Balasingham, H.Y. Jeong, W. G. Hong, H.B.-R. Lee, B. H. Kim, Y. Jun, One-step hydrothermal synthesis of graphene decorated V2O5 nanobelts for enhanced electrochemical energy storage. Sci. Rep. 5, 8151:1-8151:8 (2015). [CrossRef]
- J. Wu, X. Gao, H. Yu, T. Ding, Y. Yan, B. Yao, X. Yao, D. Chen, M. Liu, L. Huang, A scalable free-standing V2O5/CNT film electrode for supercapacitors with a wide operation voltage (1.6 V) in an aqueous electrolyte. Adv. Funct. Mater. 26, 6114-6120 (2016). [CrossRef]
- S. Gupta, B. Aberg, S. B. Carrizosa, N. Dimakis, Vanadium Pentoxide Nanobelt-Reduced Graphene Oxide Nanosheet Composites as High-Performance Pseudocapacitive Electrodes: ac Impedance Spectroscopy Data Modeling and Theoretical Calculations. Materials 9, 615:1-615:20 (2016). [CrossRef]
- S. D. Perera, A. D. Liyanage, N. Nijem, J. P. Ferraris, Y. J. Chabal, K. J. Balkus Jr. Vanadium oxide nanowire–Graphene binder free nanocomposite paper electrodes for supercapacitors: A facile green approach. J. Power Sources 230, 130-137 (2013). [CrossRef]
- H.H. Kristoffersen, H. Meitu, Structure and oxidizing power of single layer -V2O5. Topics in Catalysis, 59, 809-816 (2016). [CrossRef]
- A.M. Engstrom, F.M. Doyle, Exploring the cycle behavior of electrodeposited vanadium oxide electrochemical capacitor electrodes in various aqueous environments. J. Power Sources 228, 120-131 (2013). [CrossRef]
- J. W. Lee, S.Y. Lim, H.M. Jeong, T.H. Hwang, J.K. Kang, J.W. Choi, Extremely stable cycling of ultra-thin V2O5 nanowire–graphene electrodes for lithium rechargeable battery cathodes. Energy Environ. Sci. 5, 9889-9894 (2012). [CrossRef]
- Q. T. Qu, Y.S. Zhu, X.W. Gao, Y.P. Wu, Core-shell structure of polypyrrole grown on V2O5 nanoribbon as high-performance anode material for supercapacitors. Adv. Energy Mater. 2, 950-955 (2012). [CrossRef]
- M. Yu, Y. Zeng, Y. Han, X. Cheng, W. Zhao, C. Liang, Y. Tong, H. Tang, X. Lu, Valence-optimized vanadium oxide supercapacitor electrodes exhibit ultrahigh capacitance and super-long cyclic durability of 100 000 cycles. Adv. Funct. Mater. 25, 3534-3540 (2015). [CrossRef]
- S. Gupta, N. Dimakis, Computational predictions Computational predictions of electronic properties of graphene with defects, adsorbed transition metal-oxides and water using density functional theory. Appl. Surf. Sci. 467-468, 760-772 (2017) and references therein. [CrossRef]
- P. Simon, Y. Gogotsi, Materials for electrochemical capacitors. Nat. Mater. 7 (2008) 845–854 (2008) and references therein. [CrossRef]
- J. Han, S. Yuan, L. Liu, X. Qiu, H. Gong, X. Yang, C. Li, Y. Hao, B. Cao, Fully indium-free flexible Ag nanowires/ZnO: F composite transparent conductive electrodes with high haze. J. Mater. Chem. A3, 5375-5384 (2015). [CrossRef]
- L. Wang, H. Yang, X. Liu, R. Zeng, M. Li, Y. Huang, X. Hu, Constructing hierarchical tectorum-like α-Fe2O3/PPy nanoarrays on carbon cloth for solid-state asymmetric supercapacitors. Angew. Chem. Int. Ed. 56, 1105-1110 (2017). [CrossRef]
- Y. Zhong, X. Zhang, Y. He, H. Peng, G. Wang, G. Xin, Simultaneously armored and active graphene for transparent and flexible supercapacitors. Adv. Funct. Mater. 28, 1801998- (2018). [CrossRef]
- W. Li, C. Tan, M. A. Lowe, H. D. Abruňa, D. C. Ralph, Electrochemistry of Individual Monolayer Graphene Sheets. ACS Nano 5, 2264-2270 (2011). [CrossRef]
- S. Gupta, S. B. Carrizosa, Insights into electrode/electrolyte interfacial processes and the effect of nanostructured cobalt oxides loading on graphene-based hybrids by scanning electrochemical microscopy. Appl. Phys. Lett. 109, 243903-243905 (2016) and references therein. [CrossRef]
- J. -L. Xu, Y.-H. Liu, X. Gao, Y. Sun, S. Shen, X. Cai, L. Chen, S.-D. Wang, Embedded Ag grid electrodes as current collector for ultraflexible transparent solid-state supercapacitor. ACS Appl. Mater. Interfaces 9, 27649-27656 (2017). [CrossRef]
- X. Rui, Y. Tang, O. I. Malyi, A. Gusak, Y. Zhang, Z. Niu, H.T. Tan, C. Persson, X. Chen, Z. Chen, Q. Yan, Ambient dissolution–recrystallization towards large-scale preparation of V2O5 nanobelts for high-energy battery applications. Nano Energy 22, 583-593 (2016). [CrossRef]
- C. X. Guo, K. Sun, J. Ouyang, X. Lu, Layered V2O5/PEDOT nanowires and ultrathin nanobelts fabricated with a silk reelinglike process. Chem. Mater. 27, 5813-5819 (2015). [CrossRef]
- W. Avansi Jr, C. Ribeiro, E.R. Leite, V.R. Mastelaro, Vanadium Pentoxide Nanostructures: An Effective Control of Morphology and Crystal Structure in Hydrothermal Conditions. Cryst. Growth Des. 9, 3626-3631 (2009). [CrossRef]
- C. Xiong, A.E. Aliev, B. Gnade and K.J. Balkus Jr. Fabrication of Silver Vanadium Oxide and V2O5 Nanowires for Electrochromics. ACS Nano 2, 293-301 (2008). [CrossRef]
- E. H. Lee, M. B. Lewis, P. J. Blau, L. K. Mansur, Improved surface properties of polymer materials by multiple ion beam treatment. J. Mater. Res. 6, 610-628 (1991). [CrossRef]
- B.H. Kim, W. G. Hong, H. R. Moon, S. M. Lee, J. M. Kim, S. Kang, Y. Jun, H. J. Kimet, Investigation on the existence of optimum interlayer distance for H2 uptake using pillared-graphene oxide. Int. J. Hydrogen Energy 37, 14217-14222 (2012). [CrossRef]
- Y. Zhou, Q. Bao, L.A.L. Tang, Y. Zhong, K.P. Loh, Hydrothermal Dehydration for the “Green” Reduction of Exfoliated Graphene Oxide to Graphene and Demonstration of Tunable Optical Limiting Properties. Chem. Mater. 21, 2950-2956 (2009). [CrossRef]
- D. R. Dreyer, S. Park, C.W. Bielawski, R. S. Ruoff, The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228-240 (2010). [CrossRef]
- R. Baddour-Hadjean, V. Golabkan, J. Pereira-Ramos, A. Mantoux, D. Lincot, A Raman study of the lithium insertion process in vanadium pentoxide thin films deposited by atomic layer deposition. J. Raman Spectrosc. 33, 631-638 (2002). [CrossRef]
- A. Schaarschmidt, A.A. Farah, A. Aby, A.S. Helmy, Influence of nonadiabatic annealing on the morphology and molecular structure of PEDOT−PSS films. J. Phys. Chem. B 113, 9352-9355 (2009). [CrossRef]
- Z. Liu, K. Parvez, R. Li, R. Dong, X. Feng, K. Müllen, Transparent conductive electrodes from graphene/PEDOT:PSS hybrid inks for ultrathin organic photodetectors. Adv. Mater. 27, 669-675 (2015). [CrossRef]
- S. Kiruthika, C. Sow, G.U. Kulkarni, Transparent and flexible supercapacitors with networked electrodes. Small 13, 1701906:1-1701906:9 (2017). [CrossRef]
- J. J. Yoo, K. Balakrishnan, J. Huang, V. Meunier, B. G. Sumpter, A. Srivastava, M. Conway, A. L. M. Reddy, J. Yu, R. Vajtai, P.M. Ajayan, Ultrathin planar graphene supercapacitors. Nano Lett. 11, 1423-1427 (2011). [CrossRef]
- D. Li, W. Zhou, Q. Zhou, G. Ye, T. Wang, J. Wu, Y. Chang, J. Xu, Transparent 1T-MoS2 nanofilm robustly anchored on substrate by layer-by-layer self-assembly and its ultra-high cycling stability as supercapacitors. Nanotechnology 28, 395401:1-395401:7 (2017). [CrossRef]
- X. Y. Liu, Y.Q. Gao, G.W. Yang, A flexible, transparent, and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 8, 4227-4235 (2016). [CrossRef]
- Y. Wang, W. Zhou, Q. Kang, J. Chen, Y. Li, X. Feng, D. Wang, Y. Ma, W. Huang, Patterning islandlike MnO2 arrays by breath-figure templates for flexible transparent supercapacitors. ACS Appl. Mater. Interfaces 10, 27001-27008 (2018). [CrossRef]
- S. Bellani, L. Najafi, G. Tullii, A. Ansaldo, R. O.-Nuñez, M. Prato, M. Colombo, M. R. Antognazza, F. Bonaccorso, ITO nanoparticles break optical transparency/high-areal capacitance trade-off for advanced aqueous supercapacitors. J. Mater. Chem. A 5, 25177-25186 (2017). [CrossRef]
- E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Kluwer Academic/Plenum: New York, USA (1999).
- A. J. Bard, M.V. Mirkin (Eds.) Scanning Electrochemical Microscopy; Marcel Dekker: New York, NY, USA (2001).
- E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 101, 19-28 (1979). [CrossRef]
- D. Fraggedakis, M. McEldrew, R. B. Smith, Y. Krishnan, Y. Zhang, P. Bai, William C. Chueh, Y. S-. Horn, M. Z. Bazantet, Theory of coupled ion-electron transfer kinetics. Electroch. Acta, 367, 137432:1-137432:17 (2021). [CrossRef]
- L. Wang, H. Yang, X. Liu, R. Zeng, M. Li, Y. Huang, X. Hu, Constructing hierarchical tectorum-like α-Fe2O3/PPy nanoarrays on carbon cloth for solid-state asymmetric supercapacitors. Angew. Chem. Int. Ed. 56, 1105-1110 (2017). [CrossRef]
- H. Gerischer, in Physical Chemistry: An Advanced Treatise; H. Eyring, D. Henderson, W. Jost (Eds.) Academic Press Inc. Vol. 9A, New York (1970).
- L. M. Torres, A. F. Gil, L. Galicia, I. González, Understanding the Difference between Inner- and Outer-Sphere Mechanisms: An Electrochemical Experiment. J. Chem. Educ. 73, 808-810 (1996). [CrossRef]
- S. Gupta, B. Evans, A. Henson, S. B. Carrizosa, Salt-Assisted Ultrasonicated De-Aggregation and Advanced Redox Electrochemistry of Detonation Nanodiamond. Materials 10, 1292:1-1292:19 (2017) and references therein. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



