Submitted:
31 December 2022
Posted:
11 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
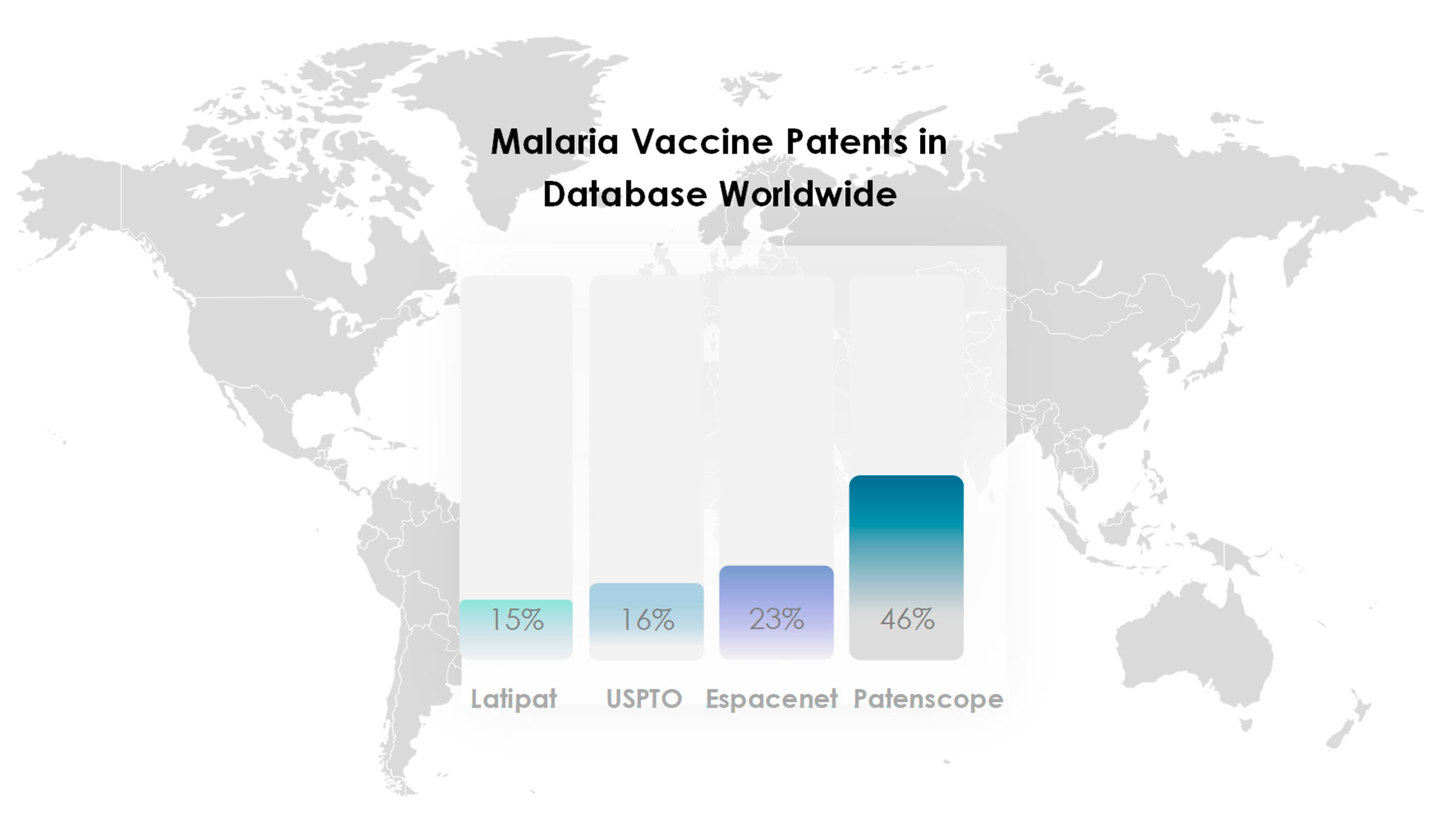
2. Search strategy and selection criteria
3. Results
3.1. Challenge of Developing a Malaria Vaccine
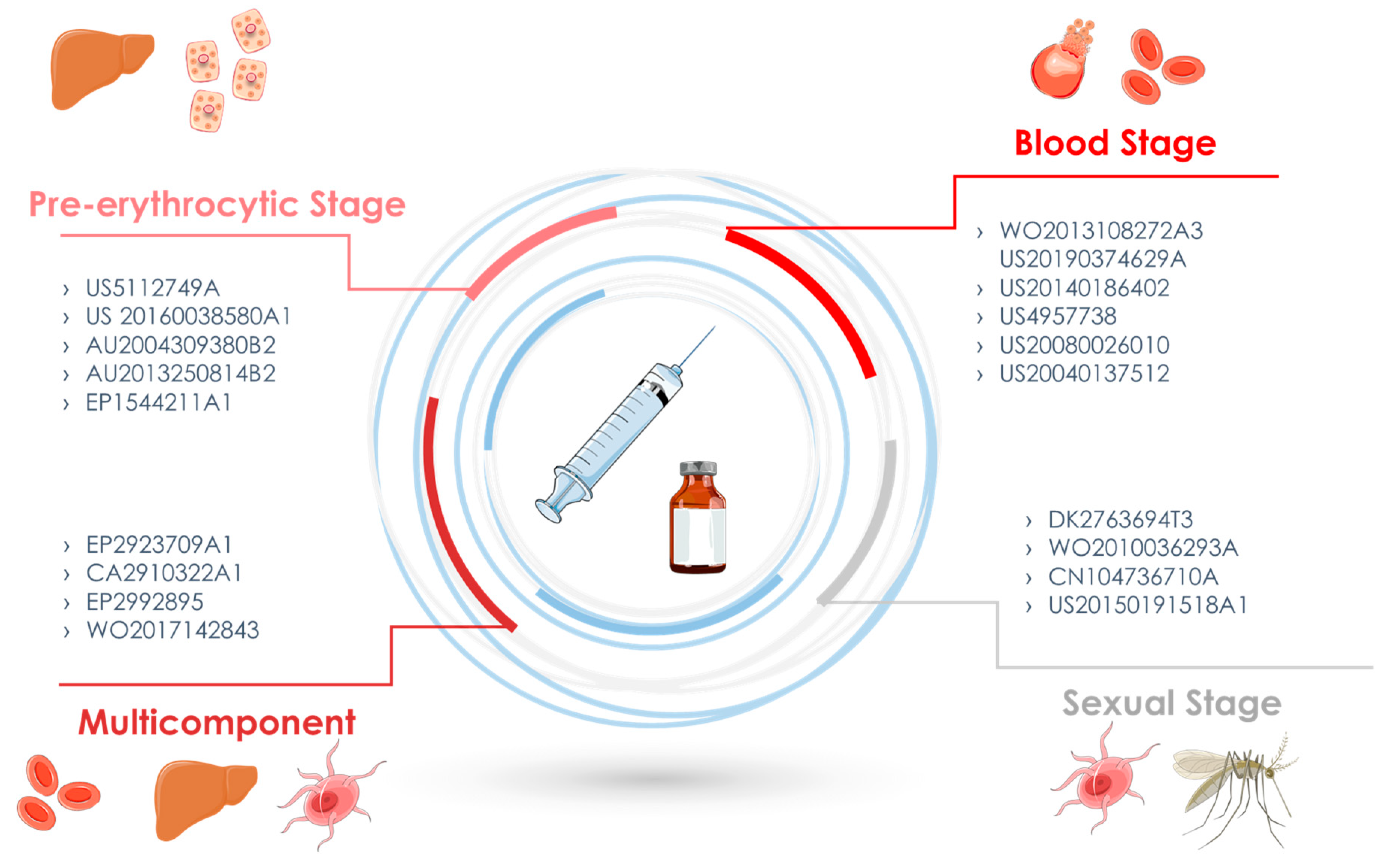
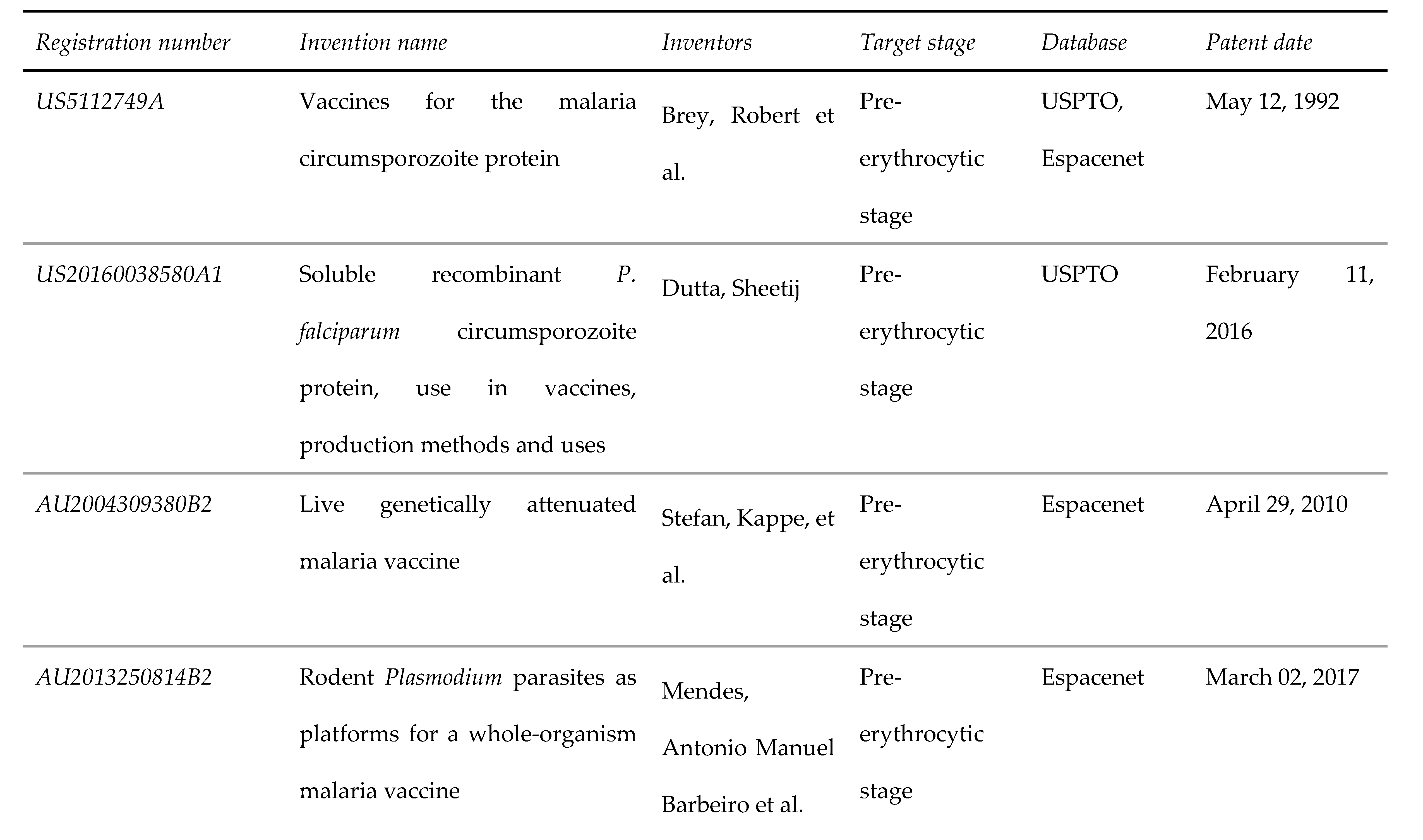
3.2. Pre-erythrocytic stage
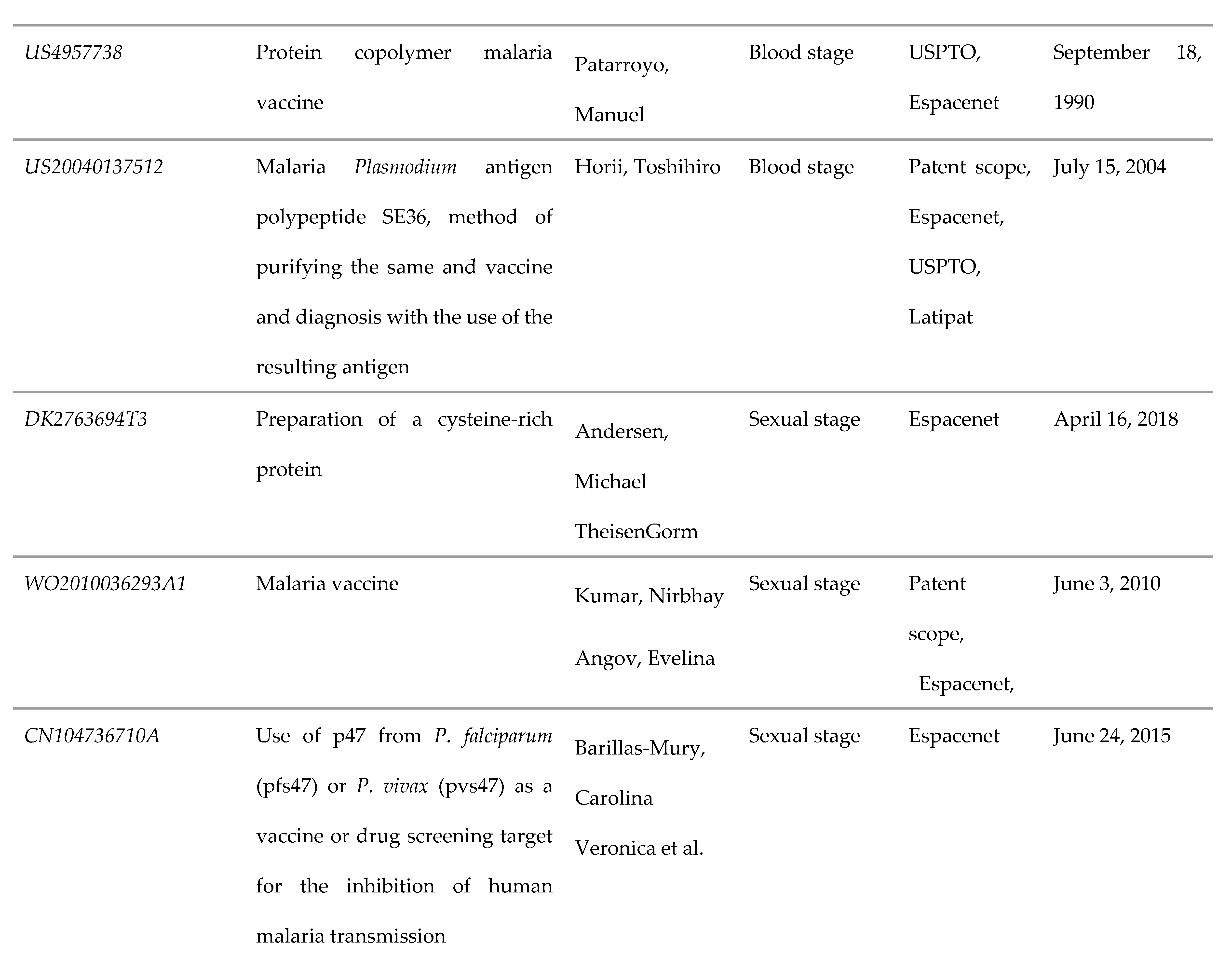
3.3. Blood Stage
3.4. Sexual Stage
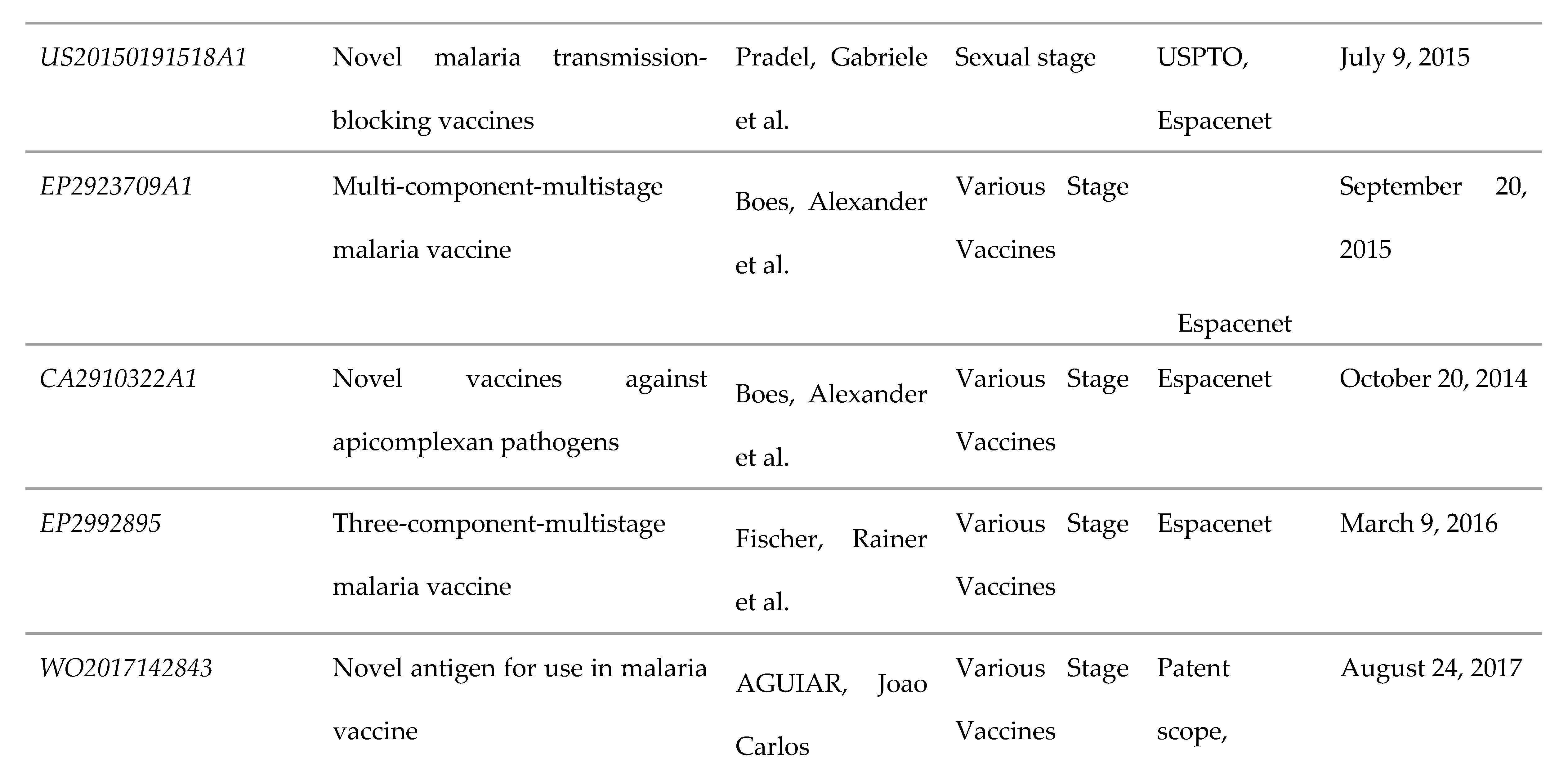
3.5. Multicomponent and/or Various Stage Vaccines
4. New approaches
5. Conclusion
Author Contributions
Acknowledgments
Conflict of Interest Statement
References
- WORLD MALARIA REPORT 2009/WHO- World Health Organization. WHO Library Cataloguing-in-Publication Data. World Malaria Report 2009. World Health Organization, 78 p. ISBN 978 92 4 156390 1. Accessed September 17, 2019.
- WORLD MALARIA REPORT 2018/WHO- World Health Organization. WHO Library Cataloguing-in-Publication Data. World Malaria Report 2018. World Health Organization, 210 p. ISBN 978 92 4 156565 3. Accessed September 17, 2019.
- Herrera S, Corradin G, Arévalo-Herrera M. An update on the search for a Plasmodium vivax vaccine. Trends in Parasitology 2007;23:122–8. [CrossRef]
- Varo R, Chaccour C, Bassat Q. Update on malaria. Medicina Clínica 2020;155:395–402. [CrossRef]
- Nilsson SK, Childs LM, Buckee C, Marti M. Targeting Human Transmission Biology for Malaria Elimination. PLoS Pathog 2015;11:e1004871. [CrossRef]
- Meibalan E, Marti M. Biology of Malaria Transmission. Cold Spring Harb Perspect Med 2017;7. [CrossRef]
- Ramaprasad A, Pain A, Ravasi T. Defining the protein interaction network of human malaria parasite Plasmodium falciparum. Genomics 2012;99:69–75. [CrossRef]
- Coker M, Folayan MO, Michelow IC, Oladokun RE, Torbunde N, Sam-Agudu NA. Things must not fall apart: the ripple effects of the COVID-19 pandemic on children in sub-Saharan Africa. Pediatr Res 2021;89:1078–86. [CrossRef]
- Sands, P. HIV, tuberculosis, and malaria: how can the impact of COVID-19 be minimised? The Lancet Global Health 2020;8:e1102–3. [CrossRef]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002;419:498–511. [CrossRef]
- Ballou, WR. Malaria vaccines in development. Expert Opinion on Emerging Drugs 2005;10:489–503. [CrossRef]
- Hill AVS. Vaccines against malaria. Phil Trans R Soc B 2011;366:2806–14. [CrossRef]
- Teixeira C, Gomes R. Experimental models in vaccine research: malaria and leishmaniasis. Braz J Med Biol Res 2013;46:109–16. [CrossRef]
- Ramjanee S, Robertson JS, Franke-Fayard B, Sinha R, Waters AP, Janse CJ, et al. The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine 2007;25:886–94. [CrossRef]
- Vincke, IH. Experimental transmission of Plasmodium berghei. Indian J Malariol 1954;8:257–62.
- Carlton JM, Hayton K, Cravo PV, Walliker D. Of mice and malaria mutants: unravelling the genetics of drug resistance using rodent malaria models. Trends Parasitol 2001: 17: 236-242. [CrossRef]
- Stowers AW, Cioce V, Shimp RL, Lawson M, Hui G, Muratova O, et al. Efficacy of Two Alternate Vaccines Based on Plasmodium falciparum Merozoite Surface Protein 1 in an Aotus Challenge Trial. Infect Immun 2001;69:1536–46. [CrossRef]
- Webster K, Collins WE, Aikawa M, Brown A, Smith CD, Tegoshi T, et al. A Primate Model for Human Cerebral Malaria: Plasmodium coatneyi-Infected Rhesus Monkeys. The American Journal of Tropical Medicine and Hygiene 1992;46:391–7. [CrossRef]
- Frimpong A, Kusi KA, Ofori MF, Ndifon W. Novel Strategies for Malaria Vaccine Design. Front Immunol 2018;9:2769. [CrossRef]
- Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. The Lancet 2001;358:1927–34. [CrossRef]
- Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, et al. Safety of the RTS, S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. The Lancet 2007;370:1543–51. [CrossRef]
- Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med 2016;374:2519–29. [CrossRef]
- Adepoju P. RTS, S malaria vaccine pilots in three African countries. The Lancet 2019;393:1685. [CrossRef]
- Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest 2010;120:4168–78. [CrossRef]
- Suau R, Vidal M, Aguilar R, Ruiz-Olalla G, Vázquez-Santiago M, Jairoce C, et al. RTS,S/AS01E malaria vaccine induces IgA responses against CSP and vaccine-unrelated antigens in African children in the phase 3 trial. Vaccine 2021;39:687–98. [CrossRef]
- Duffy, P.E., Patrick Gorres, J. Malaria vaccines since 2000: progress, priorities, products. npj Vaccines 5, 48 (2020). [CrossRef]
- Sanaria – Foundation for mission statement. http://www.sanaria.com/index.php?s=38, acessado dia 27 de junho de 2021.
- Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Human Vaccines 2010;6:97–106. [CrossRef]
- Epstein JE, Tewari K, Lyke KE, Sim BKL, Billingsley PF, Laurens MB, et al. Live Attenuated Malaria Vaccine Designed to Protect Through Hepatic CD8+ T Cell Immunity. Science 2011;334:475–80. [CrossRef]
- Plotkin, SA. Vaccines: Correlates of Vaccine-Induced Immunity. CLIN INFECT DIS 2008;47:401–9. [CrossRef]
- McNamara HA, Idris AH, Sutton HJ, Vistein R, Flynn BJ, Cai Y, et al. Antibody Feedback Limits the Expansion of B Cell Responses to Malaria Vaccination but Drives Diversification of the Humoral Response. Cell Host & Microbe 2020;28:572-585.e7. [CrossRef]
- Coelho CH, Duffy PE. Unwanted Feedback: Malaria Antibodies Hinder Vaccine Boosting. Cell Host & Microbe 2020;28:504–6. [CrossRef]
- Moon JE, Ockenhouse C, Regules JA, Vekemans J, Lee C, Chuang I, et al. A Phase IIa Controlled Human Malaria Infection and Immunogenicity Study of RTS,S/AS01E and RTS,S/AS01B Delayed Fractional Dose Regimens in Malaria-Naive Adults. The Journal of Infectious Diseases 2020;222:1681–91. [CrossRef]
- Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, et al. Fractional Third and Fourth Dose of RTS,S/AS01 Malaria Candidate Vaccine: A Phase 2a Controlled Human Malaria Parasite Infection and Immunogenicity Study. J Infect Dis 2016;214:762–71. [CrossRef]
- Vaughan AM, Aly ASI, Kappe SHI. Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding. Cell Host & Microbe 2008;4:209–18. [CrossRef]
- Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJF, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009;361:468–77. [CrossRef]
- Abuga KM, Jones-Warner W, Hafalla JCR. Immune responses to malaria pre-erythrocytic stages: Implications for vaccine development. Parasite Immunol 2021;43. [CrossRef]
- Fidock DA, Pasquetto V, Gras H, Badell E, Eling W, Ballou WR, et al. Plasmodium falciparum sporozoite invasion is inhibited by naturally acquired or experimentally induced polyclonal antibodies to the STARP antigen. Eur J Immunol 1997;27:2502–13. [CrossRef]
- John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, et al. Antibodies to Pre-erythrocytic Plasmodium falciparum Antigens and Risk of Clinical Malaria in Kenyan Children. J INFECT DIS 2008;197:519–26. [CrossRef]
- Reece WHH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, et al. A CD4+ T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med 2004;10:406–10. [CrossRef]
- White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, et al. The Relationship between RTS,S Vaccine-Induced Antibodies, CD4+ T Cell Responses and Protection against Plasmodium falciparum Infection. PLoS ONE 2013;8:e61395. [CrossRef]
- Weiss WR, Jiang CG. Protective CD8+ T lymphocytes in Primates Immunized with Malaria Sporozoites. PLoS ONE 2012;7:e31247. [CrossRef]
- Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, Takagi A, et al. Protracted Sterile Protection with Plasmodium yoelii Pre-erythrocytic Genetically Attenuated Parasite Malaria Vaccines Is Independent of Significant Liver-Stage Persistence and Is Mediated by CD8 + T Cells. J INFECT DIS 2007;196:608–16. [CrossRef]
- Oakley MS, Verma N, Zheng H, Anantharaman V, Takeda K, Gao Y, et al. Molecular Markers of Radiation Induced Attenuation in Intrahepatic Plasmodium falciparum Parasites. PLoS ONE 2016;11:e0166814. [CrossRef]
- Annoura T, Ploemen IHJ, van Schaijk BCL, Sajid M, Vos MW, van Gemert G-J, et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine 2012;30:2662–70. [CrossRef]
- Sisquella X, Nebl T, Thompson JK, Whitehead L, Malpede BM, Salinas ND, et al. Plasmodium falciparum ligand binding to erythrocytes induce alterations in deformability essential for invasion. ELife 2017;6:e21083. [CrossRef]
- Chen L, Lopaticki S, Riglar DT, Dekiwadia C, Uboldi AD, Tham W-H, et al. An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by Plasmodium falciparum. PLoS Pathog 2011;7:e1002199. [CrossRef]
- El Bissati K, Zufferey R, Witola WH, Carter NS, Ullman B, Ben Mamoun C. The plasma membrane permease PfNT1 is essential for purine salvage in the human malaria parasite Plasmodium falciparum. Proceedings of the National Academy of Sciences 2006;103:9286–91. [CrossRef]
- Graciano RCD, Ribeiro JAT, Macêdo AKS, de S. Lavareda JP, de Oliveira PR, Netto JB, et al. Recent Patents Applications in Red Biotechnology: A Mini-Review. BIOT 2019;13:170–86. [CrossRef]
- Sauerwein RW, Eling WMC. Sexual and Sporogonic Stage Antigens. In: Perlmann P, Troye-Blomberg M, editors. Chemical Immunology and Allergy, vol. 80, Basel: KARGER; 2002, p. 188–203. [CrossRef]
- Saxena AK, Wu Y, Garboczi DN. Plasmodium P25 and P28 Surface Proteins: Potential Transmission-Blocking Vaccines. Eukaryot Cell 2007;6:1260–5. [CrossRef]
- Pradel, G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology 2007;134:1911–29. [CrossRef]
- Coutinho-Abreu IV, Ramalho-Ortigao M. Transmission blocking vaccines to control insect-borne diseases: a review. Mem Inst Oswaldo Cruz 2010;105:1–12. [CrossRef]
- Eksi S, Czesny B, van Gemert G-J, Sauerwein RW, Eling W, Williamson KC. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 2006;61:991–8. [CrossRef]
- Nikolaeva D, Draper SJ, Biswas S. Toward the development of effective transmission-blocking vaccines for malaria. Expert Rev Vaccines 2015;14:653–80. [CrossRef]
- Carter R. Transmission blocking malaria vaccines. Vaccine 2001;19:2309–14. [CrossRef]
- Van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell 2001;104:153–64. [CrossRef]
- Roeffen W, Teelen K, van As J, vd Vegte-Bolmer M, Eling W, Sauerwein R. Plasmodium falciparum: Production and Characterization of Rat Monoclonal Antibodies Specific for the Sexual-Stage Pfs48/45 Antigen. Experimental Parasitology 2001;97:45–9. [CrossRef]
- Simon N, Lasonder E, Scheuermayer M, Kuehn A, Tews S, Fischer R, et al. Malaria Parasites Co-opt Human Factor H to Prevent Complement-Mediated Lysis in the Mosquito Midgut. Cell Host & Microbe 2013;13:29–41. [CrossRef]
- Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel®, a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naïve Adults. PLoS ONE 2016;11:e0163144. [CrossRef]
- Sagara I, Healy SA, Assadou MH, Gabriel EE, Kone M, Sissoko K, et al. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. The Lancet Infectious Diseases 2018;18:969–82. [CrossRef]
- Coelho CH, Rappuoli R, Hotez PJ, Duffy PE. Transmission-Blocking Vaccines for Malaria: Time to Talk about Vaccine Introduction. Trends in Parasitology 2019;35:483–6. [CrossRef]
- Standaert B, Rappuoli R. 3. How comprehensive can we be in the economic assessment of vaccines? Journal of Market Access & Health Policy 2017;5:1336044. [CrossRef]
- Duffy PE, Sahu T, Akue A, Milman N, Anderson C. Pre-erythrocytic malaria vaccines: identifying the targets. Expert Review of Vaccines 2012;11:1261–80. [CrossRef]
- Belachew EB. Immune Response and Evasion Mechanisms of Plasmodium falciparum Parasites. Journal of Immunology Research 2018;2018:1–6. [CrossRef]
- Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents 2020;55:105924. [CrossRef]
- Singh A. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat Nanotechnol 2021;16:16–24. [CrossRef]
- Wilson DS, Hirosue S, Raczy MM, Bonilla-Ramirez L, Jeanbart L, Wang R, et al. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nature Mater 2019;18:175–85. [CrossRef]
- Kanoi BN, Nagaoka H, Morita M, Tsuboi T, Takashima E. Leveraging the wheat germ cell-free protein synthesis system to accelerate malaria vaccine development. Parasitology International 2021;80:102224. [CrossRef]
- Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, et al. Wheat Germ Cell-Free System-Based Production of Malaria Proteins for Discovery of Novel Vaccine Candidates. Infect Immun 2008;76:1702–8. [CrossRef]
- Rui E, Fernandez-Becerra C, Takeo S, Sanz S, Lacerda MV, Tsuboi T, et al. Plasmodium vivax: comparison of immunogenicity among proteins expressed in the cell-free systems of Escherichia coli and wheat germ by suspension array assays. Malar J 2011;10:192. [CrossRef]
- Jaroentomeechai, T., Stark, JC, Natarajan, A. et al. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat Commun 9, 2686 (2018). [CrossRef]
- Avci FY, Li X, Tsuji M, Kasper DL. Carbohydrates and T cells: A sweet twosome. Seminars in Immunology 2013;25:146–51. [CrossRef]
- Goddard-Borger ED, Boddey JA. Implications of Plasmodium glycosylation on vaccine efficacy and design. Future Microbiology 2018;13:609–12. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





