1. Introduction
Hearing thresholds in the mid-to-high frequencies (i.e., between 1 and 4 kHz) contribute the most to understanding speech [
1]. Despite hearing aids adequately compensate for the hearing loss at these frequencies [
2], a substantial proportion of the population leave their hearing difficulties untreated. It is estimated that 63% of the people with hearing loss have never used a hearing aid [
3]; and from those fitted with hearing aids, approximately 20% do not use them at all, and 30% use them some of the time [
4]. Some reasons for refusal to wear hearing aids include denial or inadequate perception of their hearing difficulties [
5], stigma [
6], hearing-aid value and comfort [
7].
A growing body of research supports that restoring audibility does not remove all the hearing difficulties associated with hearing loss, and that hearing-aid users often complaint of experiencing high levels of listening effort and fatigue in everyday life [
8]. Consequently, the mismatch between the hearing experience of hearing aid users and their expectations imposes important levels of frustration [
4], which puts them at risk of discontinuing their use of hearing aids.
The present research aims to characterise the behavioural and neurophysiological benefits of hearing aids technologies, in an attempt to provide clinicians and audiologists with the evidence required to adequately manage the expectations of their clients, thus indirectly reinforcing hearing-aid adoption within the adult population with hearing loss.
To achieve this goal, a comprehensive test battery including both behavioural and neurophysiological assessment was administered to an adult population with and without hearing loss. This test battery considered the sensitivity of methodologies reported in previous literature to speech comprehension, as well as factors associated with hearing aids outcomes [
9].
Behavioural measures consisted of both auditory, speech comprehension and cognitive performance. Auditory testing was evaluated via modulation detection thresholds (MDT) [
10], and the spectral-temporally modulated ripple test (SMRT) [
11]. These two metrics were selected for being representative of the temporal and spectral characteristics of speech comprehension which relies on the accurate hearing detection of both the envelope and the fine structure of rapidly fluctuating acoustic information [
12]. Speech comprehension was assessed in terms of the National Acoustic Laboratories Dynamic Comprehension Test (NAL-DCT) [
13] – a validated test for speech-in-noise comprehension.
Cognitive measures included three metrics: (i) working memory which refers to the neural processes in which information is encoded and processed into meaningful units [
14]; (ii) cognitive spare capacity – refers to the residual capacity existing when all the cognitive resources have been utilized for processing a signal, most often when the signal is degraded in the presence of noise [
15]; and (iii) selective and switching attention particularly relevant in complex listening situations such as those with competing noise sources or with different speakers [
13,
16].
Neurophysiological testing consisted of a passive listening task in which the brain response was evaluated. This was an important inclusion to ensure that the non-auditory aspects such as motivation and attention do not influence the results, often a consideration during behavioural assessments. The evaluation of evoked and induced activity was considered, as the evoked (or phase locked) activity is associated with the hearing detection of the auditory stimulus this being a cortical auditory evoked potential (CAEP); and the induced (or non-phase locked) response, particularly enhanced alpha oscillations (measured as the energy in the 8–12 Hz frequency band). Alpha oscillations are a metric of selective attention to speech, inhibition of distractions and/or listening effort [
17].
Consistent with previous literature, we anticipated that hearing aids would restore the audibility of individuals with hearing loss, and consequently, we hypothesized that the two groups would present a similar performance in the auditory tests, provided that the cognitive performance in the two groups was equivalent. Naturally, should any group present a cognitive advantage over the other, the auditory tests would be difficult to compare. In regards the neurophysiological test, we hypothesized that the normal hearing (NH) and hearing loss (HL) groups would present cortical responses of similar amplitude; and that the induced response would provide useful insights on the neurophysiological processes associated with the cognitive processing of the auditory stimulus.
2. Methods
2.1. Ethics
This study was conducted at the Australian Hearing Hub (Sydney, Australia) with ethics approval from the Macquarie University Human Research Ethics Committee (Ref: 5201600438).
2.2. Participants
Ten individuals with acquired bilateral symmetric mild-moderate to moderately-severe sensorineural hearing loss (HL: 7 females, 21—68 years, mean ± std = 40.6 ±19.6 years) and ten normal hearing individuals with no concerns about listening in noise (NH: 8 females, 19—62 years, mean ± std = 35.7 ± 14.0 years) participated in the study. The difference between the age distributions from the two groups was not statistically significant (p=0.53). All HL participants were regular bilateral hearing aid users for at least five years and wore digital hearing aids fitted with NAL-NL2 prescription targets.
Participant selection criteria included: (i) an “A” type tympanogram, (ii) an acoustic reflex thresholds within 70—100 dB hearing level range, (iii) bilateral and symmetrical hearing loss defined as ≤20 dB between the four-frequency average hearing loss (i.e. the average of the hearing thresholds at 0.5, 1, 2 and 4 kHz frequencies), and (iv) a Montreal Cognitive Assessment (MoCA) [
18] score ≥26.
2.3. Procedure
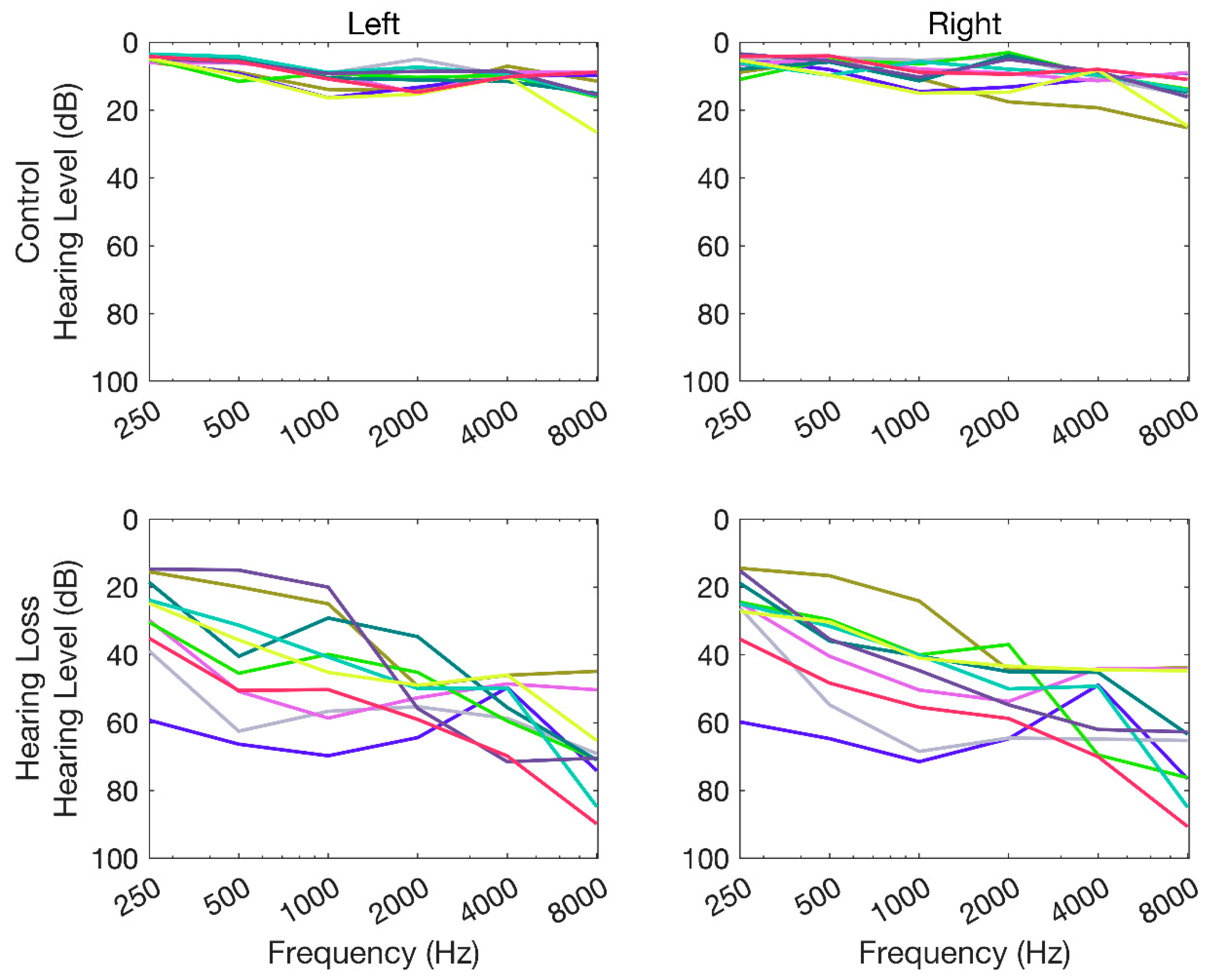
Pure-tone audiometry was carried out using Interacoustics audiometer AC 40 (Interacoustics, Denmark) for octave frequencies between 0.25 to 8 kHz. All the participants in the NH group had their hearing thresholds within 20 dB hearing level.
For all tests conducted under headphones and earphones, the acoustic signal of the auditory stimulus was modified according to the NAL-RP (NAL-Revised Profound) prescription formula via Matlab (The Mathworks Inc., Natick, MA) to ensure adequate audibility [
19]. In order to test individuals with normal hearing, the same filter with zero gain was applied.
For tests that required verbal presentation of the stimuli and stimuli presentation through loudspeaker, a standard hearing aid (GN Resound’s Linx quattro 9) with NAL-RP gain was used to ensure uniformity across all participants The NAL-RP prescription formula was chosen for the current study for the following reasons: (i) It is a standard approach that has been widely used in other research studies [
20]; (ii) the formula has been systematically assessed to ensure adequate performance [
21]; and (iii) it has similar gain at moderate inputs similar to other hearing aid prescriptions such as NAL-NL1 or NAL-NL2 [
22]. For the HL participants, the gain was determined based on the hearing loss. The following steps were taken: (i) measurement of the real-ear aided response; (ii) measurement of the unaided response; and (iii) calculation of the real ear insertion gain, i.e., the difference between real ear aided and unaided response.
The CAEP for a speech detection task was measured in quiet.
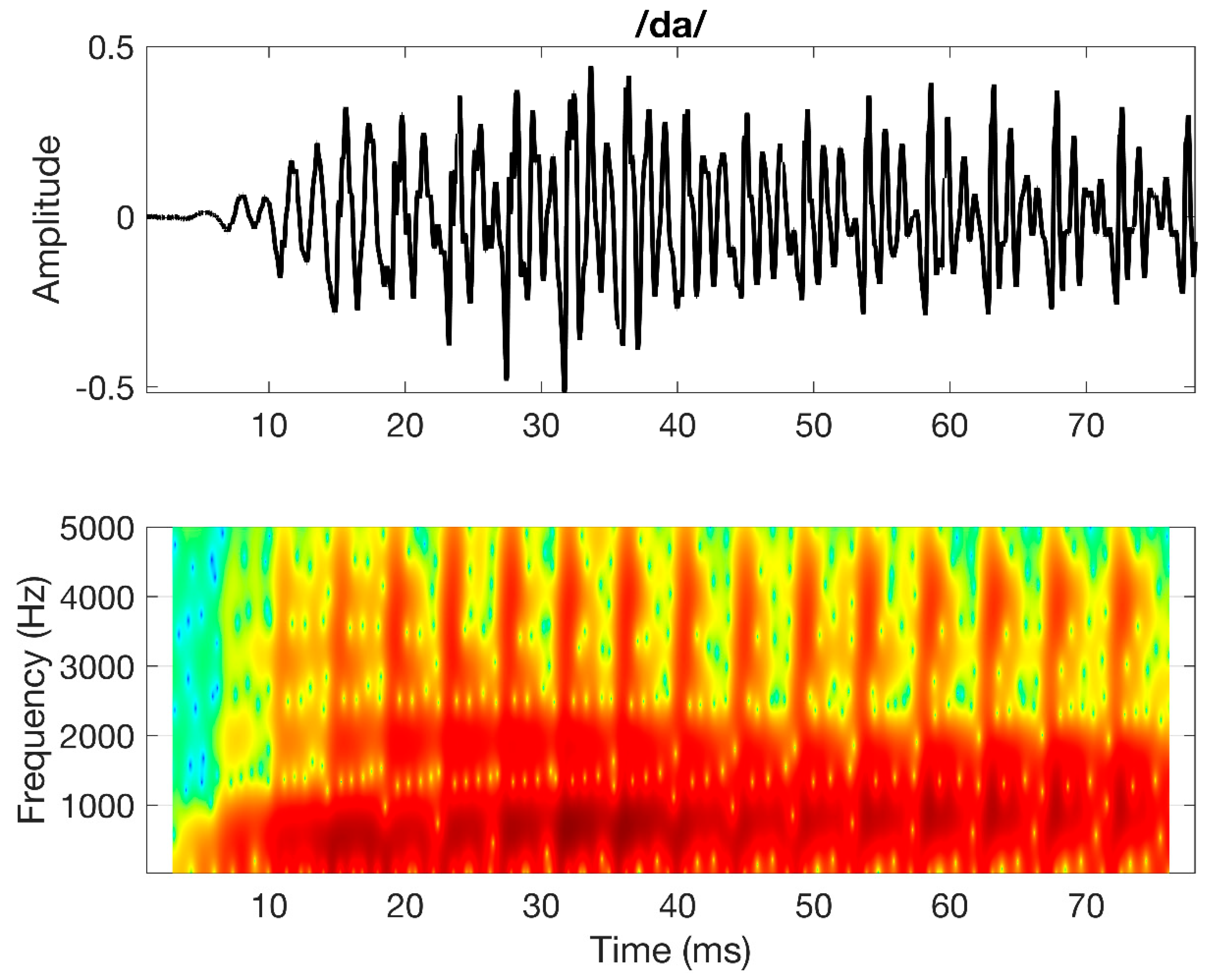
Figure 1 presents the auditory stimulus used in the neurophysiological test. This stimulus consists of a 79 ms duration /da/ naturally spoken by an Australian female speaker. The stimulus was presented diotically by ER-3A insert transducers compatible with the Neuroscan system (Compumedics limited, Abbotsford, Australia). The number of stimulus repetitions was 150 to ensure a robust evoked response and the inter-stimulus interval was jittered between 950 and 1100 ms. The stimulus was presented at 65 dB SPL in quiet. The task of the individual consisted of staying alert and attend to the stimuli and to avoid head movements. Calibration of the /da/ stimulus was done using a Brüel and Kjær (B & K) type 2250 G4 (Brüel and Kjær Sound and Vibration Measurement A/S, Nærum, Denmark) sound level meter connected to an artificial ear Type 4152, using a 1-inch pressure microphone Type 4144, a 2cc coupler BD 1038 and an oscilloscope. The NAL-RP filters were applied to provide necessary amplification while testing individuals with hearing loss.
The EEG recording was carried out in an acoustically and electromagnetically shielded room at the Australian Hearing Hub facilities of Macquarie University (Sydney, Australia). All participants were seated on a comfortable chair and were requested to remain still during the testing. Sixty-four channel electroencephalography (EEG) recording was carried out using NeuroscanR Acquire 4.5 (Compumedics, Germany) using the International 10/20 EEG system. The left mastoid electrode was used as the reference electrode (M1). Online filtering of 0.01-100 Hz was used. The horizontal and vertical movements of the eye were also measured using electrodes placed on outer canthus of the eye for horizontal movements, above and below the eye for vertical movements.
Table 1 provides a brief description of the auditory tests included in this study, i.e., MDT and SMRT. Speech-in-noise comprehension was assessed using the NAL-DCT [
13]. Before starting the NAL-DCT task, an adaptive sentence in noise test was carried out using the Beautifully Efficient Speech Test (BEST) [
23] in the presence of simulated cafeteria noise comprising seven two-talker conversations at 65 dB sound pressure level (SPL) [
24] to obtain the SRT for each participant. Cognitive processing tests included digit-span test which is a subtest of Wechsler Adult Intelligence Scale IV [
25], elevator task with distraction and reversal subtest of Test of Everyday Attention [
26], and Cognitive Spare Capacity Test (CSCT) [
13]. The auditory stimuli of these tests were created using custom made scripts in Matlab and presented through a Focusrite 219 sound card (Focusrite, England) to HDA 300 Sennheiser headphones (Senheiser, Germany) using a sampling rate of 44100 Hz. For all the auditory tests three alternative-forced-choice (AFC) adaptive tracking was used. The threshold was calculated by taking the average of the last six reversals. Each test had three runs of each condition to ensure that they had a good test-retest reliability.
Data Analysis
The analysis of the behavioural data obtained comprised a multivariate analysis of variance (MANOVA) using age as a co-variate. The behavioural data of the 10 individuals in the HL group was compared to the 10 individuals from NH group.
The CAEP recordings were analysed offline using custom made scripts using the Fieldtrip toolbox in Matlab. The continuous EEG signal was first re-referenced to the common average reference after excluding noisy electrode channels which were visually identified. The re-referenced data were then epoched between -100 to 500 ms range relative to the onset of the stimulus, and baseline corrected considering the time range -100 to 0 ms. Artefacts originating from eye movements were removed using independent component analysis which uses a blind source separation approach a mathematic processing that identifies the components with maximal temporal statistical independency. The EEG signals were then subjected to digital band-pass filter between 0.1 to 30 Hz. A variance rejection criterion was applied to identify noisy trials. All trials with variances above 80 µV [
2] were excluded from the averaging process. The accepted trials were used to obtain a CAEP waveform and for time frequency analysis.
The global field power (GFP) was calculated for the two groups. GFP helps to quantify the variability of the neural activity across the scalp as a function of time, thus constituting a single reference-independent measure of the response strength [
27].
The P1-N1-P2 complex from the NH and HL groups were compared via a non-parametric randomization procedure to overcome multiple electrode comparison effects. An independent t-test was used as the statistical test to evaluate the differences between the NH and HL groups. The output from the statistical test consisted of a cluster of electrodes, and the p-values were estimated according to Monte Carlo simulations. Only the clusters that comprised of cluster values greater than 95% among all the clusters derived from random permutation of data were considered. The Inter-trial phase coherence (ITPC) measures the consistencies of the phase responses in the time-frequency region. The ITPC was used to explain which time-frequency regions are associated with the evoked responses.
Time frequency analysis, a wavelet transform function was used to convert the accepted trials to the time-frequency domain. The wavelet transform function utilizes the Mortlet wavelet with a filter tap of five cycles in-order to ensure a good time-frequency resolution. Following this, these time-frequency spectra were then converted to a relative power measure known as event related spectral perturbation (ERSP) [
28]. The ERSP represents the change in brain oscillatory activities. Monte-Carlo cluster permutation analysis slope
t-test was performed across and within the groups to determine if there were any differences.
3. Results
Figure 2 presents the audiometric thresholds for the NH and HL groups. This figure shows that the participants from the HL group (bottom plots) had a bilateral mild-moderate to moderately severe sensorineural hearing loss. All participants in the NH group (top plots) had audiometric thresholds below 20 dB hearing level in all tested frequencies except for one participant with an audiometric threshold of 25 dB HL at 8 kHz.
Table 2 shows the mean, median and standard deviation for the HL and NH group respectively to the auditory (rows 1 and 2), cognitive (rows 3 to 7) and speech-comprehension (row 8) tests. A MANOVA analysis with Bonferroni correction applied showed similar performance between the NH and HL groups in all the behavioural tests.
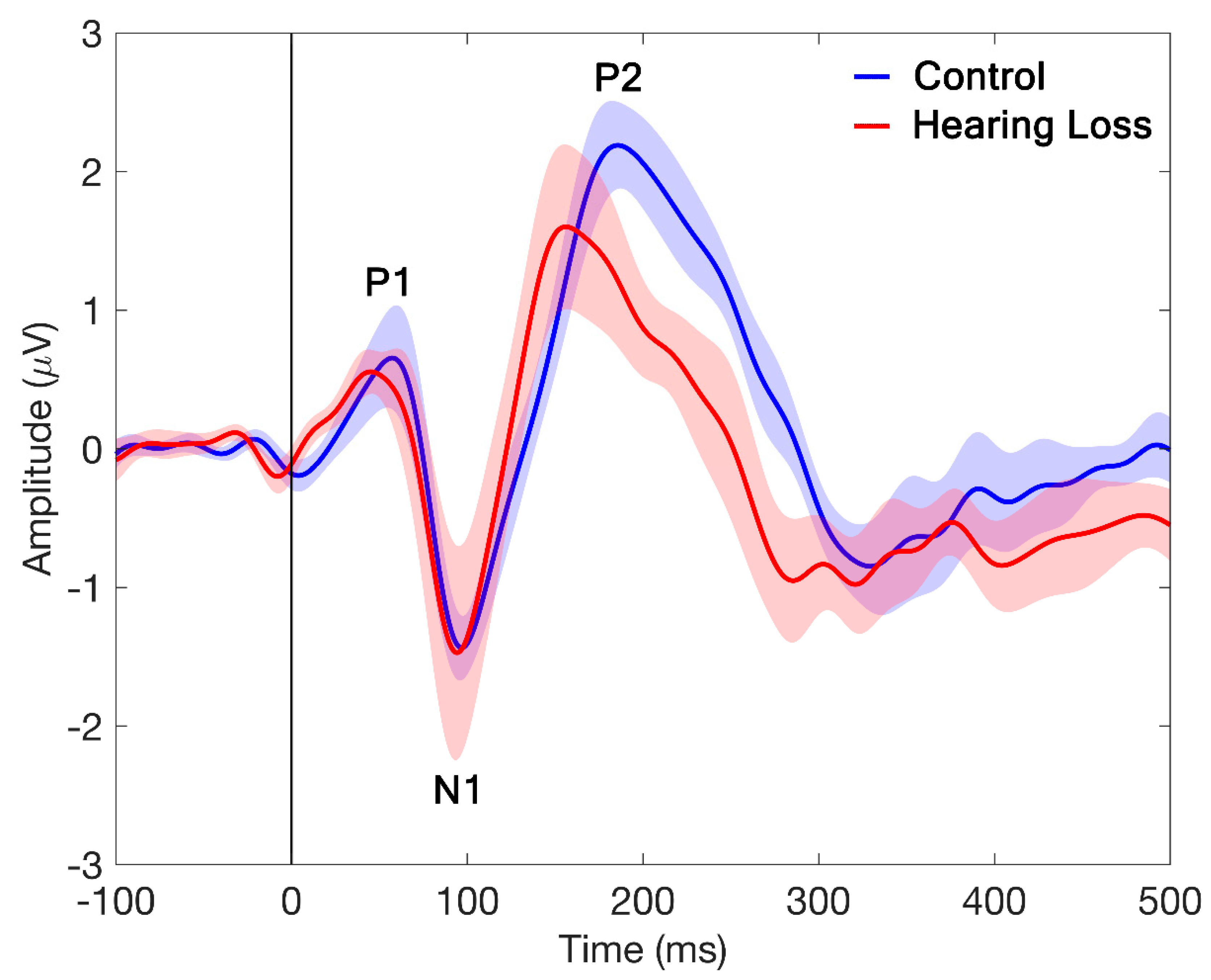
Figure 3 shows the grand average waveforms obtained for the NH and HL groups for the fronto-central electrodes (15 electrodes including F3, F1, Fz, F2, F4, FC3, FC1, FCz, FC2, FC4, C3, C1, Cz, C2, C4) comparing /da/-evoked P1-N1-P2 responses. An Independent samples t-test carried out on CAEP amplitudes showed no significant differences between the NH and HL groups (t (17) =1.0, p-value = 0.3).
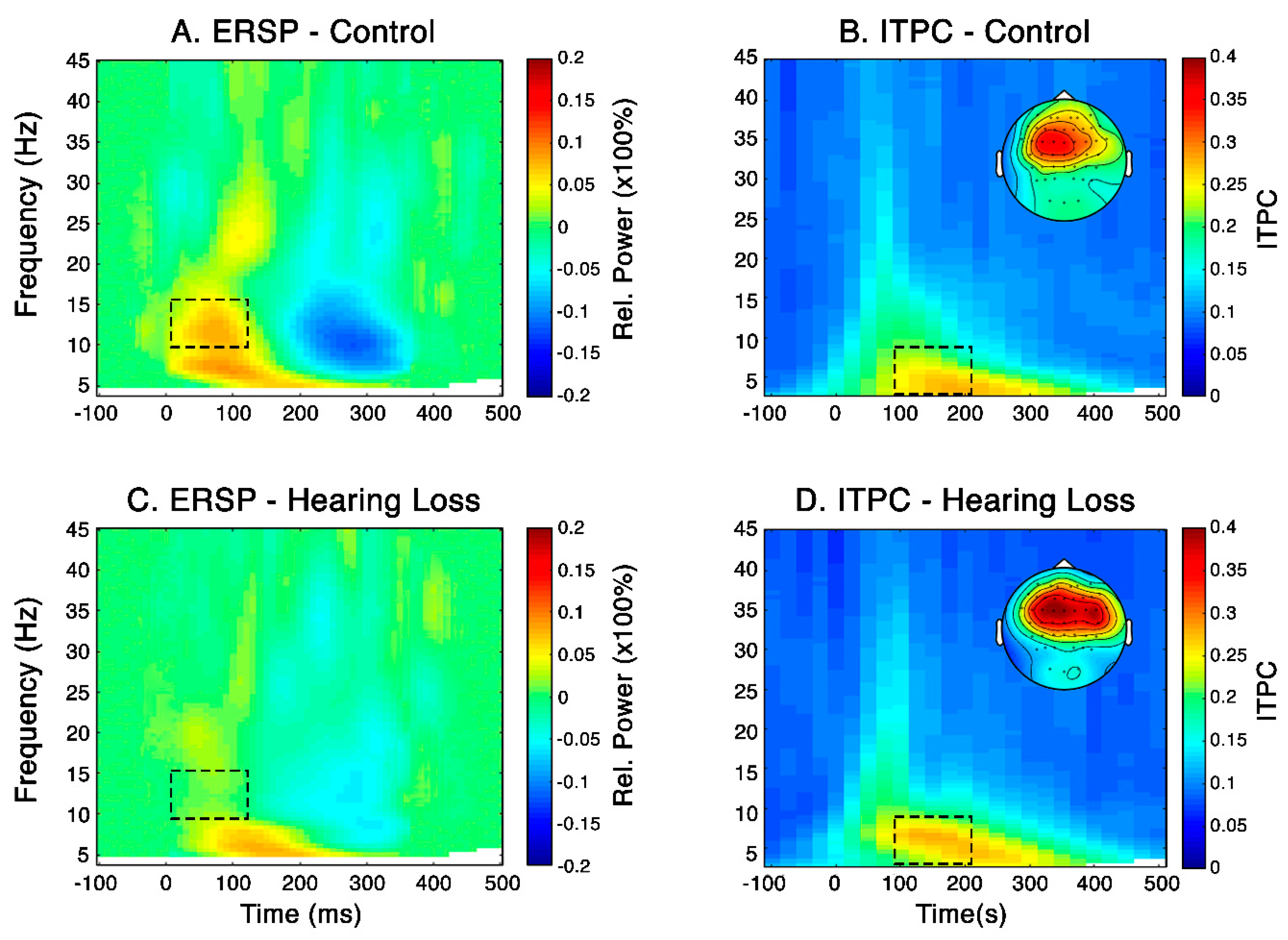
Subplots B and D in
Figure 4 present the ITPC obtained in the NH (control) and HL groups, respectively, which was used to map the evoked responses in the time-frequency plane across subjects. Consistent with the CAEP (
Figure 3), cluster permutation statistics showed that the specified timing and frequency ITPC measure is significantly different to that of the baseline for the two groups (p-value = 0.002). ITPC showed that the timing and frequencies of the phase-locked brain responses were located between 80 to 220 ms and 4 to 7 Hz.
Subplots A and C in
Figure 4 present the time-frequency analysis conducted in the two groups aimed at investigating their induced response. These subplots show that the NH group presented a statistically significant increase in synchronised alpha oscillations (8-12 Hz) relative to the HL group (p=0.039) at the time interval 25-125 ms in centro-frontal electrodes.
4. Discussion
The current study aimed at providing a comprehensive characterisation of hearing aids benefits, including both behavioural and neurophysiological testing. We hypothesized that should the two groups presented similar cognitive capacities, hearing aids would effectively compensate for the audibility of individuals with hearing loss, and they would present a similar performance than the normal hearing group in the auditory and speech comprehension tasks. Due to the compensated audibility in the HL group, we also hypothesized that the evoked cortical responses of the two groups would have a similar amplitude, and that the alpha power analysis in the induced response would inform on higher processing levels of the auditory stimulus.
The present study showed no differences in performance between the two groups on any of the cognitive tests, which ensured compatible cognitive capacities between the two groups thus enabling their comparison in terms of auditory and speech-in-noise comprehension. This finding is also consistent with previous literature investigating cognitive performance in hearing aid users [
29].
Consistent with our prediction, results showed no statistically significant differences between the NH and HL groups in any of the auditory or speech-in-noise comprehension tests. This finding supports that when the auditory stimulus is presented at the required level, experienced hearing aid users are able to perform like the NH group on auditory-related tasks, including speech-in-noise comprehension.
This study showed that the CAEP signals obtained with the NH and HL groups presented similar amplitudes, consistent with the literature [
30]. This result is consistent with our expected outcomes in which hearing aids would restore audibility in individuals with hearing loss; and supports the benefits of the long-term use of hearing aid technologies.
Results also revealed a statistically significant difference between the NH and HL groups in the induced response at the alpha frequency band (8–12 Hz), being higher in the NH group in the centro-frontal electrodes. Previous literature has suggested that presence of increased synchronised alpha oscillations is indicative of increased selective attention and inhibition [
31]. Synchronised alpha oscillations were reported to be observed during the tasks that requires one to pay attention to the given stimuli, while not responding to them [
32]. In addition, alpha synchronization has also been observed as an inhibitory response by the different areas of the cortical regions that are not relevant to a task [
33]. For instance, large alpha power was observed over the visual cortical areas while the task required one to pay attention to the auditory stimuli [
34]. Although the EEG test did not require participants to remember or to react to the stimuli, participants were instructed to listen and focus on the stimuli.
Based on the previous literature, the enhanced alpha synchronisations found in the NH group is consistent with the hypothesis that this group is better at paying attention to the stimuli and inhibit the irrelevant distractors, irrelevant distractors in this situation could be the generic state of staying alert while not paying attention to any of the things placed in the test room compared to the HL group [
32,
34]. The differences observed in alpha oscillations between the groups requires further investigation especially in view of the fact that the HL group’s performance on all behavioural tasks and evoked activity showed similar performance to the NH group.
5. Conclusion
This is one of the first studies to include both behavioural (cognitive and auditory processing and speech-in-noise comprehension) and neurophysiological tests (including both induced and evoked responses) to evaluate the benefits of hearing aid technologies in experienced users. Results show that when audibility is compensated via hearing aids in individuals with hearing loss, (i) their behavioural performance in a wide range auditory and speech-in-noise comprehension tasks is like a NH group with equivalent cognitive skills; and (ii) the amplitude of their cortical evoked responses is similar to the NH group. Importantly, these findings provide audiologists and hearing clinicians with evidence that supports the use of hearing aids.
Results also revealed that the HL group presented a reduced alpha synchronisation compared to the NH group, which could be associated with decreased inhibition, or selective attention. The difference in induced activity exhibit variances that persist possibly indicating difficulties in terms of active listening despite appropriate audibility but require further investigation. The characterisation of this dimension of their hearing is essential to provide clinicians a complete picture of the hearing experience as hearing-aid users. This evidence may support clinicians when counselling their clients not only about the benefits of hearing aids, but also about the many challenges, thus adequately managing their expectations by helping them build realistic goals which could be measured and tracked via the Client Oriented Scale of Improvement (COSI). Ultimately, this improved counselling is expected to reduce frustration of new hearing-aid users and increase the uptake of this technology.
Acknowledgements
The authors acknowledge the financial support of the HEARing CRC, established under the Australian Government’s Cooperative Research Centres (CRC) Program and by the Australian Government Department of Health. This work is a part of the doctoral thesis of the first author at Macquarie University. The authors thank Dr. Varghese Peter (Macquarie University, Sydney, Australia) for his guidance in the analyses, and Prof. Robert Cowan (HEARing Cooperative Research Centre, Melbourne, Australia) for his in-depth editorial to the manuscript.
Conflicts of Interest
The authors declare no competing conflicts of interest.
References
- Van Rooij, J.C.; Plomp, R. Auditive and cognitive factors in speech perception by elderly listeners. III. Additional data and final discussion. The Journal of the Acoustical Society of America 1992, 91, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B. The future of hearing aid technology. Trends in amplification 2007, 11, 31–45. [Google Scholar] [CrossRef]
- Bisgaard, N.; Ruf, S. Findings from EuroTrak surveys from 2009 to 2015: Hearing loss prevalence, hearing aid adoption, and benefits of hearing aid use. American journal of audiology 2017, 26, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Linssen, A.M., Joore, M.A., Minten, R.K.H et al. Qualitative interviews on the beliefs and feelings of adults towards their ownership, but non-use of hearing aids. International Journal of Audiology 2013, 52, 670–677. [CrossRef] [PubMed]
- Rawool, V. Denial by patients of hearing loss and their rejection of hearing health care: a review. Journal of Hearing Science 2018, 8. [Google Scholar] [CrossRef]
- Erler, S.F.; Garstecki, D.C. Hearing loss-and hearing aid-related stigma 2002. [CrossRef]
- McCormack, A.; Fortnum, H. Why do people fitted with hearing aids not wear them? International journal of audiology 2013, 52, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Davis, H., Schulndt, D., Bonnet, K. et al. Understanding listening-related fatigue: Perspectives of adults with hearing loss. International Journal of Audiology 2020, 60, 458–468. [CrossRef]
- Saunders, G.H.; TH Chisolm and H., B. Abrams. "Measuring hearing aid outcomes-not as easy as it seems." Journal of rehabilitation research and development 2005, 42c, 157.
- Feng, Y., Yin, S., Kiefte, M. et al. Temporal resolution in regions of normal hearing and speech perception in noise for adults with sloping high-frequency hearing loss. Ear and hearing 2010, 31, 115–125. Ear and hearing 2010, 31, 115–125. [CrossRef]
- Won, J.H.; Drennan, W.R.; Rubinstein, J.T. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. Journal of the Association for Research in Otolaryngology 2007, 8, 384–392. [Google Scholar] [CrossRef]
- Hopkins, K.; Moore, B.C. Development of a fast method for measuring sensitivity to temporal fine structure information at low frequencies. International Journal of Audiology 2010, 49, 940–946. [Google Scholar] [CrossRef]
- Keidser, G., Best, V., Freeston, K et al. Cognitive spare capacity: evaluation data and its association with comprehension of dynamic conversations. Frontiers in psychology 2015, 6, 597. [CrossRef]
- Baddeley, A. (1992). Working memory. Science 255, 5044, 556-559.
- Mishra, S., Lunner, T., Stenfelt, S. et al. Visual information can hinder working memory processing of speech 2013. [CrossRef]
- Shinn-Cunningham, B. G., & Best, V. (2008). Selective attention in normal and impaired hearing. Trends in amplification 2008, 12, 283–299. [CrossRef]
- Obleser, J.; Weisz, N. Suppressed alpha oscillations predict intelligibility of speech and its acoustic details. Cerebral cortex 2012, 22, 2466–2477. [Google Scholar] [CrossRef]
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005, 53, 695–699. [CrossRef] [PubMed]
- Byrne, D.; Upfold, G. Implications of ear canal resonance for hearing aid fitting. In Seminars in Hearing 1991, 12, 34–41. [Google Scholar] [CrossRef]
- Glyde, H.; Cameron, S.; Dillon, H. The effects of hearing impairment and aging on spatial processing. Ear and hearing 2013, 34, 15–28. [Google Scholar] [CrossRef]
- Byrne, D.; Cotton, S. Evaluation of the National Acoustic Laboratories' new hearing aid selection procedure. Journal of Speech, Language, and Hearing Research 1988, 31, 178–186. [Google Scholar] [CrossRef]
- Byrne, D., Dillon, H., Ching, T. NAL-NL1 procedure for fitting nonlinear hearing aids: characteristics and comparisons with other procedures. Journal of the American Academy of Audiology 2001; 12. [CrossRef]
- Best, V.; M McLelland, & H. Dillon. The BEST (Beautifully Efficient Speech Test) for Evaluating Speech Intelligibility in Noise. World Congress of Audiology Brisbane 2014, Australia.
- Keidser, G., Dillon, H., Mejia, J. et al. An algorithm that administers adaptive speech-in-noise testing to a specified reliability at selectable points on the psychometric function. International Journal of Audiology 2013, 52, 795–800. [CrossRef]
- Wechsler, D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson. 2008, 22, 816–827.
- Robertson, I. H., Ward, T., Ridgeway, V et al. The test of everyday attention (TEA). Bury St. Edmunds, UK: Thames Valley Test Company 1994; 197-221.
- Murray, M.M.; Brunet, D.; Michel, C.M. Topographic ERP analyses: A step-by-step tutorial review. Brain Topography 2008, 20, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Grandchamp, R.; Delorme, A. Single-trial normalization for event-related spectral decomposition reduces sensitivity to noisy trials. Frontiers in psychology 2011, 2, 236. [Google Scholar] [CrossRef]
- Glick, H.A.; Sharma, A. Cortical neuroplasticity and cognitive function in early-stage, mild-moderate hearing loss: Evidence of neurocognitive benefit from hearing aid use. Frontiers in neuroscience 2020, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Koerner, T. K., & Zhang, Y. (2018). Differential effects of hearing impairment and age on electrophysiological and behavioral measures of speech in noise. Hearing research 2018, 370, 130–142. [CrossRef]
- Klimesch, W., Sauseng, P., & Hanslmayr, S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain research reviews 2007, 53, 63–88. [CrossRef]
- Jensen, O., Gelfand, J., Kounios, J. et al. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral cortex 2002, 12, 877–882. [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends in cognitive sciences 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Foxe, J.J.; Simpson, G.V.; Ahlfors, S.P. Parieto-occipital ∼10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 1998, 9, 3929–3933. [Google Scholar] [CrossRef]
Figure 1.
Signal waveform (top) and spectrogram (bottom) of the /da/ stimulus used in the physiological test.
Figure 1.
Signal waveform (top) and spectrogram (bottom) of the /da/ stimulus used in the physiological test.
Figure 2.
Pure-tone audiometric thresholds (in dB hearing level) for octave frequencies between 250 Hz to 8 kHz for hearing-loss and normal-hearing participants.
Figure 2.
Pure-tone audiometric thresholds (in dB hearing level) for octave frequencies between 250 Hz to 8 kHz for hearing-loss and normal-hearing participants.
Figure 3.
Grand-average CAEP waveforms at fronto-central electrodes (15 electrodes including F3, F1, Fz, F2, F4, FC3, FC1, FCz, FC2, FC4, C3, C1, Cz, C2, C4) from the NH and HL groups obtained at 65 dB SPL. Shadows represent standard deviation of the measures.
Figure 3.
Grand-average CAEP waveforms at fronto-central electrodes (15 electrodes including F3, F1, Fz, F2, F4, FC3, FC1, FCz, FC2, FC4, C3, C1, Cz, C2, C4) from the NH and HL groups obtained at 65 dB SPL. Shadows represent standard deviation of the measures.
Figure 4.
[A and C] Grand-average event-related spectral perturbation (ERSP) across subjects for centro-frontal electrodes (15 electrodes including F3, F1, Fz, F2, F4, FC3, FC1, FCz, FC2, FC4, C3, C1, Cz, C2, C4) for the normal hearing (NH) and hearing loss (HL) groups. The dotted squares highlight a statistically significant difference in alpha (8-12 Hz) power synchronization between the NH and HL groups. [B and D] Grand-average inter-trial phase coherence (ITPC) across subjects for all electrodes in quiet for the NH and HL groups. The dotted squares highlight the time and frequency range in which the evoked response is present; and the topographic plots show the power distribution of that section of the ITPC across electrodes.
Figure 4.
[A and C] Grand-average event-related spectral perturbation (ERSP) across subjects for centro-frontal electrodes (15 electrodes including F3, F1, Fz, F2, F4, FC3, FC1, FCz, FC2, FC4, C3, C1, Cz, C2, C4) for the normal hearing (NH) and hearing loss (HL) groups. The dotted squares highlight a statistically significant difference in alpha (8-12 Hz) power synchronization between the NH and HL groups. [B and D] Grand-average inter-trial phase coherence (ITPC) across subjects for all electrodes in quiet for the NH and HL groups. The dotted squares highlight the time and frequency range in which the evoked response is present; and the topographic plots show the power distribution of that section of the ITPC across electrodes.
Table 1.
Detailed test description of the auditory tests.
Table 1.
Detailed test description of the auditory tests.
| Test |
Stimuli |
Description |
| MDT: Conducted using The Maximum Likelihood Procedure (MLP; Grassi and Soranzo, 2009) toolbox |
Gaussian noise of 500 ms, amplitude modulated at 60 Hz |
Three alternative-forced-choice (AFC) adaptive tracking was used. Two of the stimuli presented were the reference stimuli having zero modulation and third one was the variable stimulus having the modulated signal. The threshold was calculated by taking the average of the last six reversals. Each test had three runs of the same to ensure that we had a good test-retest reliability. The task was to identify the variable stimulus. |
| |
|
|
| SMRT (Aronoff and Landsberger, 2013) |
Stimuli was created using a non-harmonic complex that consisted of 202 equal amplitude pure-tone frequency components that spaced every 1/33.3 of an octave between 100 to 6400 Hz. The duration of each stimulus was 500 ms with 10-ms linear onset and offset ramps |
Three alternative-forced-choice (AFC) adaptive tracking was used. Two stimuli presented were reference stimuli having 20 ripples per octave and third one was the variable stimuli having 2 ripples per octave. The threshold was calculated by taking the average of the last six reversals. Each test had three runs of the same to ensure that we had a good test-retest reliability. The task was to identify the variable stimuli. |
Table 2.
Mean, median and standard deviations (in brackets) of auditory and cognitive tasks for the normal hearing (NH) and hearing loss (HL) groups. The MANOVA analysis, Bonferroni corrections applied, show no statistically significant differences in performance between the two groups.
Table 2.
Mean, median and standard deviations (in brackets) of auditory and cognitive tasks for the normal hearing (NH) and hearing loss (HL) groups. The MANOVA analysis, Bonferroni corrections applied, show no statistically significant differences in performance between the two groups.
| |
NH |
HL
|
| Test |
MEAN; STDEV |
MEDIAN |
MEAN; STDEV |
MEDIAN |
MANOVA |
| MDT 60Hz (in dB) |
-13.6
1.6 |
-13.6
|
-16.1;
1.8 |
-15.9
|
[F(1, 17) = 8.4, p= 0.01 partial ŋ2 =0.3] |
| SRN (ripples per octave) |
7.0;
1.3 |
7.5 |
7.0;
1.8 |
7.8
|
[F(1, 17) = 0.0, p= 0.9, partial ŋ2 =0.0] |
| Digit span forward (raw score) |
10.6;
1.4 |
10.5 |
12.0;
2.1 |
11.5 |
[F(1, 17) = 2.6, p= 0.1, partial ŋ2 =0.1] |
| Digit span backward (raw score) |
9.3;
1.8 |
9.0 |
8.7;
2.2 |
8.0 |
[F(1, 17) = 0.4, p= 0.4, partial ŋ2 =0.02], |
| TEA: distractor (scaled score) |
11.7;
1.7 |
12.5 |
9.9;
2.2 |
10.2
|
[F(1, 17) = 3.3, p= 0.08, partial ŋ2 =0.1] |
| TEA: reversal (scaled score) |
9.9;
3.7 |
10.5 |
11.1;
2.6 |
11.5 |
[F(1, 17) = 0.8, p= 0.3, partial ŋ2 =0.04] |
| CSCT (raw score) |
8.3;
1.7 |
8.5 |
8.6;
1.5 |
9.0 |
[F(1, 17) = 0.2, p= 0.6, partial ŋ2 =0.01] |
| DCT (raw score) |
21.6;
5.4 |
22.7 |
17.0;
8.3 |
18.25 |
[F(1, 17) = 2.3, p= 0.1, partial ŋ2 =0.1] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).