Submitted:
09 January 2023
Posted:
12 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design & participants
2.2. Measurements
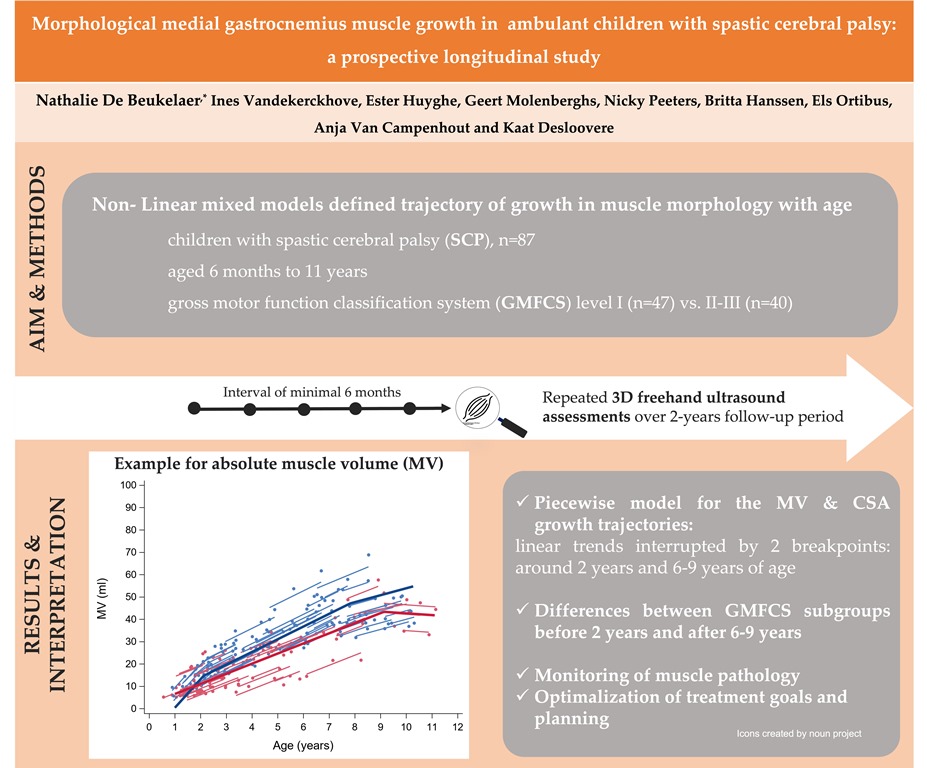
2.3. Statistical analysis
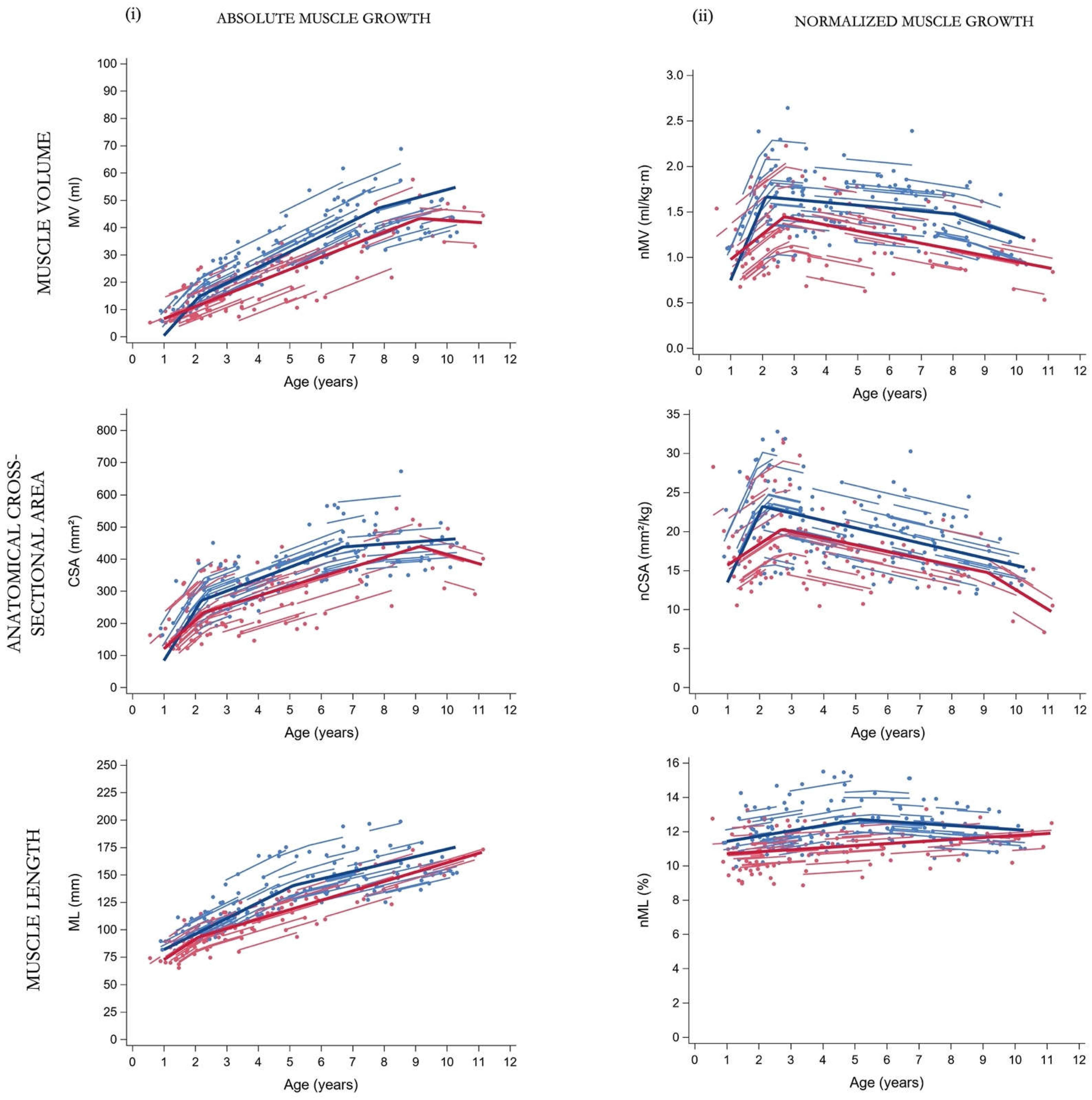
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, H. K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, Di. L.; Becher, J. G.; Gaebler-Spira, D.; Colver, A.; Reddihough, Di. S.; Crompton, K. E.; Lieber, R. L. Cerebral Palsy. Nat. Rev. Dis. Prim. 2016, 2(1), 15082. [Google Scholar] [CrossRef]
- Barrett, R. S.; Lichtwark, G. A. Gross Muscle Morphology and Structure in Spastic Cerebral Palsy: A Systematic Review. Dev. Med. Child Neurol. 2010, 52(9), 794–804. [Google Scholar] [CrossRef]
- Schless, S. H.; Cenni, F.; Bar-On, L.; Hanssen, B.; Goudriaan, M.; Papageorgiou, E.; Aertbeliën, E.; Molenaers, G.; Desloovere, K. Combining Muscle Morphology and Neuromotor Symptoms to Explain Abnormal Gait at the Ankle Joint Level in Cerebral Palsy. Gait Posture 2019, 68(December 2018), 531–537. [Google Scholar] [CrossRef]
- Rosenbaum, P. L.; Walter, S. D.; Hanna, S. E.; Palisano, R. J.; Russell, D. J.; Raina, P.; Wood, E.; Bartlett, D. J.; Galuppi, B. E. Prognosis for Gross Motor Function in Cerebral Palsy Creation of Motor Development Curves. JAMA 2002, 208(11), 1357–1363. [Google Scholar] [CrossRef]
- Nordmark, E.; Hägglund, G.; Lauge-Pedersen, H.; Wagner, P.; Westbom, L. Development of Lower Limb Range of Motion from Early Childhood to Adolescence in Cerebral Palsy: A Population-Based Study. BMC Med. 2009, 7. [Google Scholar] [CrossRef]
- Lindén, O.; Hägglund, G.; Rodby-Bousquet, E.; Wagner, P. The Development of Spasticity with Age in 4,162 Children with Cerebral Palsy: A Register-Based Prospective Cohort Study. Acta Orthop. 2019, 90(3), 286–291. [Google Scholar] [CrossRef]
- Burgess, A.; Reedman, S.; Chatfield, M. D.; Ware, R. S.; Sakzewski, L.; Boyd, R. N. Development of Gross Motor Capacity and Mobility Performance in Children with Cerebral Palsy: A Longitudinal Study. Dev. Med. Child Neurol. 2022, 64(5), 578–585. [Google Scholar] [CrossRef]
- Palisano, R. J.; Rosenbaum, P.; Bartlett, D.; Livingston, M. H. Content Validity of the Expanded and Revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50(10), 744–750. [Google Scholar] [CrossRef]
- Molenaers, G.; Fagard, K.; Van Campenhout, A.; Desloovere, K. Botulinum Toxin A Treatment of the Lower Extremities in Children with Cerebral Palsy. J.Child Orthop 2013, 7, 383–387. [Google Scholar] [CrossRef]
- Kruse, A.; Rivares, C.; Weide, G.; Tilp, M.; Jaspers, R. T. Stimuli for Adaptations in Muscle Length and the Length Range of Active Force Exertion—A Narrative Review. Front. Physiol. 2021, 12(October). [Google Scholar] [CrossRef] [PubMed]
- Verschuren, O.; Smorenburg, A. R. P.; Luiking, Y.; Bell, K.; Barber, L.; Peterson, M. D. Determinants of Muscle Preservation in Individuals with Cerebral Palsy across the Lifespan: A Narrative Review of the Literature. J. Cachexia. Sarcopenia Muscle 2018, 9(3), 453–464. [Google Scholar] [CrossRef]
- Gough, M.; Shortland, A. P. Early Muscle Development in Children with Cerebral Palsy. the Consequences for Further Muscle Growth, Muscle Function, and Long-Term Mobility; Roberta Shepherd, 2013. [CrossRef]
- Herskind, A.; Ritterband-Rosenbaum, A.; Willerslev-Olsen, M.; Lorentzen, J.; Hanson, L.; Lichtwark, G.; Nielsen, J. B. Muscle Growth Is Reduced in 15-Month-Old Children with Cerebral Palsy. Dev. Med. Child Neurol. 2016, 58(5), 485–491. [Google Scholar] [CrossRef]
- De Beukelaer, N. De; Weide, G.; Huyghe, E.; Vandekerckhove, I.; Hanssen, B.; Peeters, N.; Uytterhoeven, J.; Deschrevel, J.; Maes, K.; Corvelyn, M.; Costamagna, D.; Gayan-Ramirez, G.; Van Campenhout, A.; Desloovere, K. Reduced Cross-Sectional Muscle Growth Six Months after Botulinum Toxin Type-A Injection in Children with Spastic Cerebral Palsy. Toxins (Basel). 2022, 14(139). [Google Scholar] [CrossRef]
- Malaiya, R.; McNee, A. E.; Fry, N. R.; Eve, L. C.; Gough, M.; Shortland, A. P. The Morphology of the Medial Gastrocnemius in Typically Developing Children and Children with Spastic Hemiplegic Cerebral Palsy. J. Electromyogr. Kinesiol. 2007, 17(6), 657–663. [Google Scholar] [CrossRef]
- Barber, L.; Hastings-Ison, T.; Baker, R.; Barrett, R.; Lichtwark, G. Medial Gastrocnemius Muscle Volume and Fascicle Length in Children Aged 2 to 5 Years with Cerebral Palsy. Dev. Med. Child Neurol. 2011, 53(6), 543–548. [Google Scholar] [CrossRef]
- Oberhofer, K.; Stott, N. S.; Mithraratne, K.; Anderson, I. A. Subject-Specific Modelling of Lower Limb Muscles in Children with Cerebral Palsy. Clin. Biomech. 2010, 25(1), 88–94. [Google Scholar] [CrossRef]
- Pitcher, C. A.; Elliott, C. M.; Valentine, J. P.; Stannage, K.; Williams, S. A.; Shipman, P. J.; Reid, S. L. Muscle Morphology of the Lower Leg in Ambulant Children with Spastic Cerebral Palsy. Muscle and Nerve 2018, 58(6), 818–823. [Google Scholar] [CrossRef]
- Hanssen, B.; Peeters, N.; Vandekerckhove, I.; De Beukelaer, N.; Bar-On, L.; Molenaers, G.; Van Campenhout, A.; Degelaen, M.; Van den Broeck, C.; Calders, P.; Desloovere, K. The Contribution of Decreased Muscle Size to Muscle Weakness in Children With Spastic Cerebral Palsy. Front. Neurol. 2021, 12(July), 1–14. [Google Scholar] [CrossRef]
- Schless, S. H.; Cenni, F.; Bar-On, L.; Hanssen, B.; Kalkman, B.; O’brien, T.; Aertbeliën, E.; Van Campenhout, A.; Molenaers, G.; Desloovere, K. Medial Gastrocnemius Volume and Echo-Intensity after Botulinum Neurotoxin A Interventions in Children with Spastic Cerebral Palsy. Dev. Med. Child Neurol. 2018, 61(7), 783–790. [Google Scholar] [CrossRef]
- Noble, J. J.; Chruscikowski, E.; Fry, N. R. D.; Lewis, A. P.; Gough, M.; Shortland, A. P. The Relationship between Lower Limb Muscle Volume and Body Mass in Ambulant Individuals with Bilateral Cerebral Palsy. BMC Neurol. 2017, 17(1), 1–9. [Google Scholar] [CrossRef]
- Lieber, R. L.; Steinman, S.; Barash, I. A.; Chambers, H. Structural and Functional Changes in Spastic Skeletal Muscle. Muscle Nerve 2004, 29(5), 615–627. [Google Scholar] [CrossRef]
- Gundersen, K. Excitation-Transcription Coupling in Skeletal Muscle: The Molecular Pathways of Exercise. Biol. Rev. Camb. Philos. Soc. 2011, 86(3), 564. [Google Scholar] [CrossRef]
- Clowry, G. J. The Dependence of Spinal Cord Development on Corticospinal Input and Its Significance in Understanding and Treating Spastic Cerebral Palsy. Neuroscience and Biobehavioral Reviews. Pergamon January 1, 2007, pp 1114–1124. [CrossRef]
- Noble, J. J.; Fry, N. R.; Lewis, A. P.; Keevil, S. F.; Gough, M.; Shortland, A. P. Lower Limb Muscle Volumes in Bilateral Spastic Cerebral Palsy. 2014. [CrossRef]
- Gorter, J. W.; Tol, E. Van; Schie, P. Van; Ketelaar, M.; Palisano, R.; Rosenbaum, P.; Bartlett, D.; Livingston, M.; Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. GMFCS – E & R Gross Motor Function Classification System Expanded and Revised. 2009, 2007–2010.
- Palisano, R. J.; Cameron, D.; Rosenbaum, P. L.; Walter, S. D.; Russell, D. Stability of the Gross Motor Function Classification System. Dev. Med. Child Neurol. 2006, 48(6), 424–428. [Google Scholar] [CrossRef]
- Cenni, F.; Schless, S. H.; Bar-On, L.; Aertbeliën, E.; Bruyninckx, H.; Hanssen, B.; Desloovere, K. Reliability of a Clinical 3D Freehand Ultrasound Technique: Analyses on Healthy and Pathological Muscles. Comput. Methods Programs Biomed. 2018, 156, 97–103. [Google Scholar] [CrossRef]
- Barber, L.; Alexander, C.; Shipman, P.; Boyd, R.; Reid, S.; Elliott, C. Validity and Reliability of a Freehand 3D Ultrasound System for the Determination of Triceps Surae Muscle Volume in Children with Cerebral Palsy. J. Anat. 2019, 234(3), 384–391. [Google Scholar] [CrossRef]
- Williams, S. A.; Bell, M.; Kim, H. K.; Salim Al Masruri, G.; Stott, N. S.; Fernandez, J.; Mirjalili, S. A. The Reliability and Validity of Triceps Surae Muscle Volume Assessment Using Freehand Three-dimensional Ultrasound in Typically Developing Infants. J. Anat. 2021, (No. July), 1–12. [Google Scholar] [CrossRef]
- Thomason, P.; Willoughby, K.; Graham, H. K. Orthopaedic Assessment. In Cerebral palsy: science and clinical practice; Mac Keith Press: London, UK, 2014; pp. 287–312. [Google Scholar]
- Hanssen, B.; De Beukelaer, N.; Schless, S. H.; Cenni, F.; Bar-On, L.; Peeters, N.; Molenaers, G.; Van Campenhout, A.; Van den Broeck, C.; Desloovere, K. Reliability of Processing 3-D Freehand Ultrasound Data to Define Muscle Volume and Echo-Intensity in Pediatric Lower Limb Muscles with Typical Development or with Spasticity. Ultrasound Med. Biol. 2021, 47(9), 2702–2712. [Google Scholar] [CrossRef]
- Hanssen, B. Muscle Structure, Weakness, and Strengthening: A Complex Triangle for Children with Spastic Cerebral Palsy, KU Leuven, 2022.
- Cenni, F.; Schless, S. H.; Monari, D.; Bar-On, L.; Aertbeliën, E.; Bruyninckx, H.; Hanssen, B.; Desloovere, K. An Innovative Solution to Reduce Muscle Deformation during Ultrasonography Data Collection. J. Biomech. 2018, 77, 194–200. [Google Scholar] [CrossRef]
- Treece, G.; Prager, R.; Gee, A.; Berman, L. Fast Surface and Volume Estimation from Non-Parallel Cross-Sections, for Freehand Three-Dimensional Ultrasound. Med. Image Anal. 1999, 3(2), 141–173. [Google Scholar] [CrossRef]
- Handsfield, G. G.; Meyer, C. H.; Hart, J. M.; Abel, M. F.; Blemker, S. S. Relationships of 35 Lower Limb Muscles to Height and Body Mass Quantified Using MRI. J. Biomech. 2014, 47(3), 631–638. [Google Scholar] [CrossRef]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models in Practice: A SAS-Oriented Approach; Springer: New York, 1997. [Google Scholar]
- Molenberghs, G.; Verbeke, G. An Introduction to (Generalized) (Non-)Linear Mixed Models. In Explantory im respons models: A generalized linear and nonlinear approach; De Boeck, P., Wilson, M., Eds.; Springer-Verlag: New York, 2004; pp 111–153.
- Jaric, S.; Mirkov, D.; Markovic, G. Normalizing Physical Performance Tests for Body Size: A Proposal for Standardization. J. Strength Cond. Res. 2005, 19(2), 467–474. [Google Scholar] [CrossRef]
- Wells, J. C. K.; Davies, P. S. W.; Fewtrell, M. S.; Cole, T. J. Body Composition Reference Charts for UK Infants and Children Aged 6 Weeks to 5 Years Based on Measurement of Total Body Water by Isotope Dilution. Eur. J. Clin. Nutr. 2020, 74(1), 141–148. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.; Hastings-Ison, T.; Baker, R.; Kerr Graham, H.; Barrett, R.; Lichtwark, G. The Effects of Botulinum Toxin Injection Frequency on Calf Muscle Growth in Young Children with Spastic Cerebral Palsy: A 12-Month Prospective Study. J. Child. Orthop. 2013, 7(5), 425–433. [Google Scholar] [CrossRef]
- Day, S. M.; Strauss, D. J.; Vachon, P. J.; Rosenbloom, L.; Shavelle, R. M.; Wu, Y. W. Growth Patterns in a Population of Children and Adolescents with Cerebral Palsy. Dev. Med. Child Neurol. 2007, 49(3), 167–171. [Google Scholar] [CrossRef] [PubMed]
- de las Mercedes Ruiz Brunner, M.; Cuestas, E.; Heinen, F.; Schroeder, A. S. Growth in Infants, Children and Adolescents with Unilateral and Bilateral Cerebral Palsy. Sci. Rep. 2022, 12(1). [Google Scholar] [CrossRef]
- Bénard, M. R.; Harlaar, J.; Becher, J. G.; Huijing, P. A.; Jaspers, R. T. Effects of Growth on Geometry of Gastrocnemius Muscle in Children: A Three-Dimensional Ultrasound Analysis. J. Anat. 2011, 219(3), 388–402. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R. L.; Fridén, J. Functional and Clinical Significance of Skeletal Muscle Architecture. Muscle Nerve 2000, 23(November), 1647–1666. [Google Scholar]
- Hägglund, G.; Wagner, P. Spasticity of the Gastrosoleus Muscle Is Related to the Development of Reduced Passive Dorsiflexion of the Ankle in Children with Cerebral Palsy: A Registry Analysis of 2,796 Examinations in 355 Children. Acta Orthop. 2011, 82(6), 744–748. [Google Scholar] [CrossRef]
- Mathewson, M. A.; Lieber, R. L. Pathophysiology of Muscle Contractures in Cerebral Palsy. Phys. Med. Rehabil. Clin. N. Am. 2015, 26(1), 57–67. [Google Scholar] [CrossRef]
- Williams, I.; Reid, L.; Stott, N. S.; Valentine, J.; Elliott, C. Measuring Skeletal Muscle Morphology and Architecture with Imaging Modalities in Children with Cerebral Palsy: A Scoping Review. Dev. Med. Child Neurol. 2020, 63(3), 263–273. [Google Scholar] [CrossRef]
- Park, E.-Y.; Kim, W.-H. Meta-Analysis of the Effect of Strengthening Interventions in Individuals with Cerebral Palsy. Res. Dev. Disabil. 2014, 35, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Anker–van der Wel, I.; Smorenburg, A. R. P.; de Roos, N. M.; Verschuren, O. Dose, Timing, and Source of Protein Intake of Young People with Spastic Cerebral Palsy. Disabil. Rehabil. 2020, 42(15), 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, B.; Peeters, N.; De Beukelaer, N.; Vannerom, A.; Peeters, L.; Molenaers, G.; Van Campenhout, A.; Deschepper, E.; Van den Broeck, C.; Desloovere, K. Progressive Resistance Training for Children with Cerebral Palsy: A Randomized Controlled Trial Evaluating the Effects on Muscle Strength and Morphology. Frontiers in Physiology. 2022. [CrossRef] [PubMed]
- Alexander, C.; Elliott, C.; Valentine, J.; Stannage, K.; Bear, N.; Donnelly, C. J.; Shipman, P.; Reid, S. Muscle Volume Alterations after First Botulinum Neurotoxin A Treatment in Children with Cerebral Palsy: A 6-Month Prospective Cohort Study. Dev. Med. Child Neurol. 2018, 60(11), 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Damiano, D.; Abel, M.; Romness, M.; Oeffinger, D.; Tylkowski, C.; Gorton, G.; Bagley, A.; Nicholson, D.; Barnes, D.; Calmes, J.; Kryscio, R.; Rogers, S. Comparing Functional Profiles of Children with Hemiplegic and Diplegic Cerebral Palsy in GMFCS Levels I and II: Are Separate Classifications Needed? Dev. Med. Child Neurol. 2006, 48(10), 797–803. [Google Scholar] [CrossRef]
- Barber, L. A.; Read, F.; Lovatt Stern, J.; Lichtwark, G.; Boyd, R. N. Medial Gastrocnemius Muscle Volume in Ambulant Children with Unilateral and Bilateral Cerebral Palsy Aged 2 to 9 Years. Dev. Med. Child Neurol. 2016, 58(11). [Google Scholar] [CrossRef]
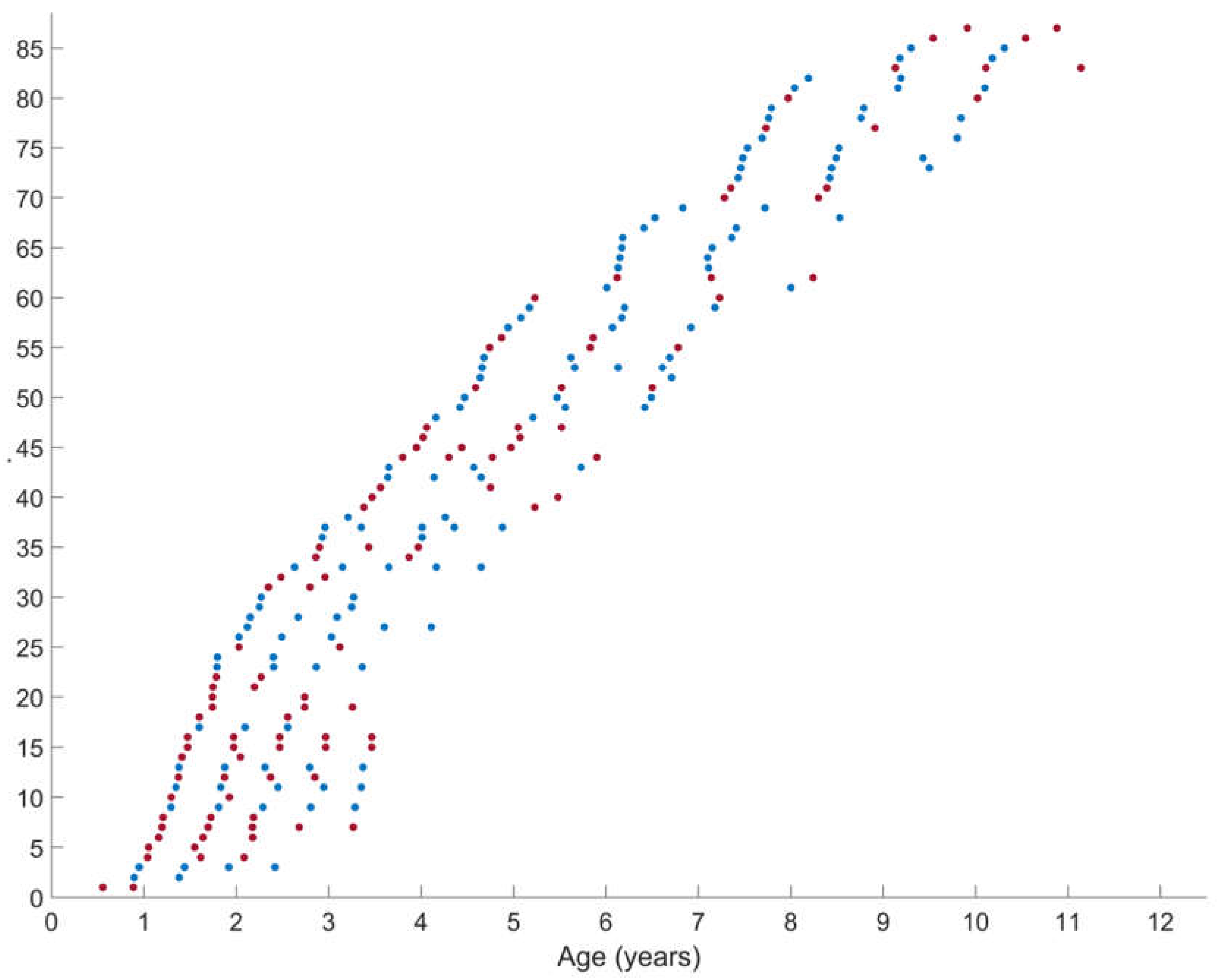
| Group | GMFCS I | GMFCS II-III |
|---|---|---|
| Participants | 47 | 40 |
| Observations | 130 | 104 |
| Sex (boy/girls) | 25 / 22 | 21 / 19 |
| GMFCS level (I /II/ III) | 47 / 0 / 0 | 0 / 22 / 18 |
| Involvement (unilateral/bilateral) | 37 / 10 | 14 (II, =10 & III=4) / 26 (II=12 & III=14) |
| History of BoNT-A injection | 11 | 7 |
| Antropometric & muscle outcomes | ||
| Age (y) | 4.66 (2.25-6.83) | 2.88 (1.47-4.81) |
| Body mass (kg) | 18.0 (13.0-22.0) | 12.5 (10.0-16.4) |
| Body length (cm) | 104.7 (88.5-121.1) | 90.0 (77.8-106.0) |
| MV (ml) | 28.2 (16.7-38.4) | 13.6 (7.4-25.5) |
| CSA (mm2) | 335.6 (260.1-392.1) | 205.5 (162.3-285.8) |
| ML (mm) | 127.1 (99.2-144.9) | 96.0 (82.8-127.1) |
| nMV (ml/kg⋅m) | 1.45 (1.26-1.58) | 1.12 (0.92-1.39) |
| nCSA (mm2/kg) | 19.4 (16.1-22.1) | 16.1 (13.8-19.0) |
| nML (%) | 11.7 (11.3-12.6) | 10.9 (9.8-11.9) |
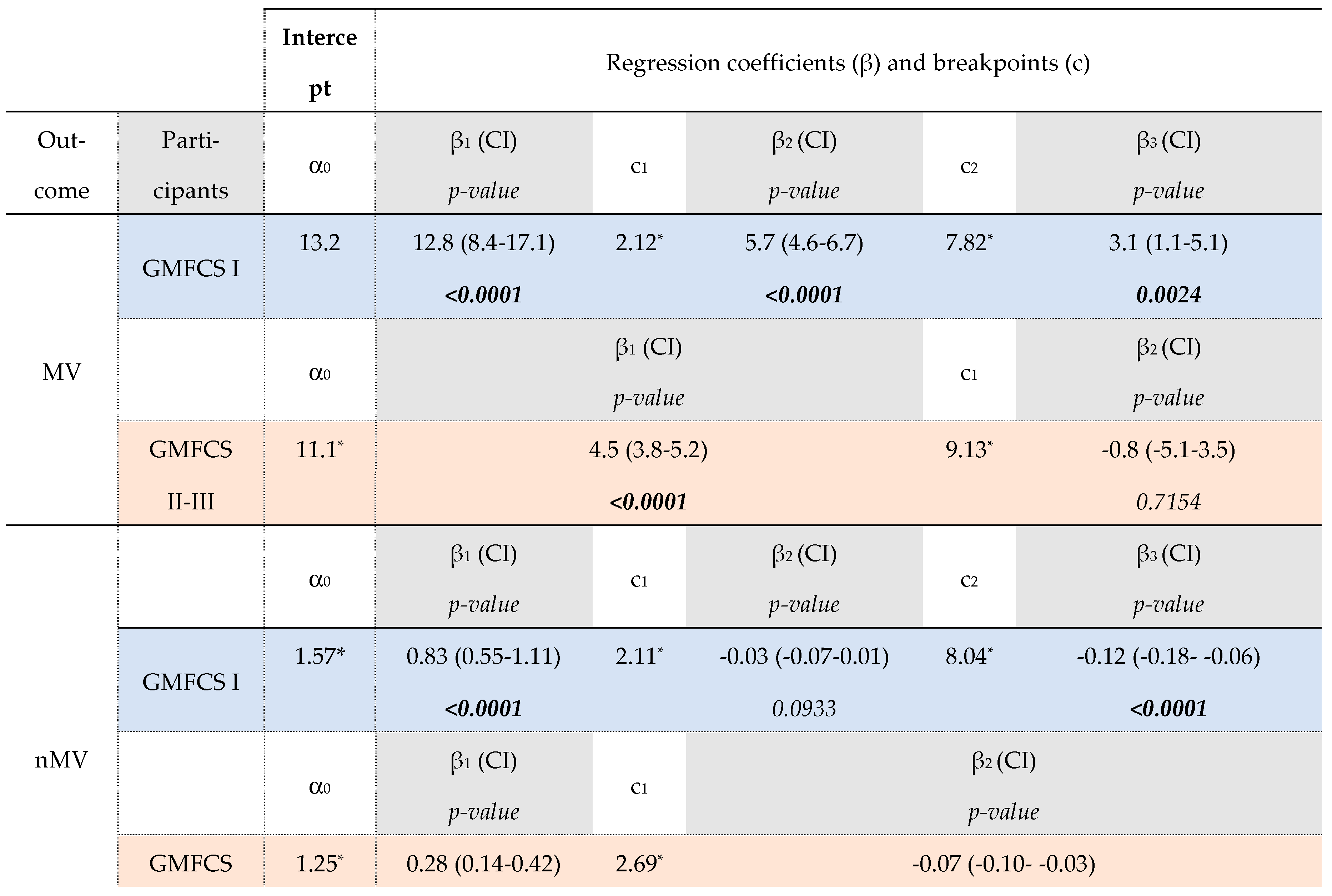 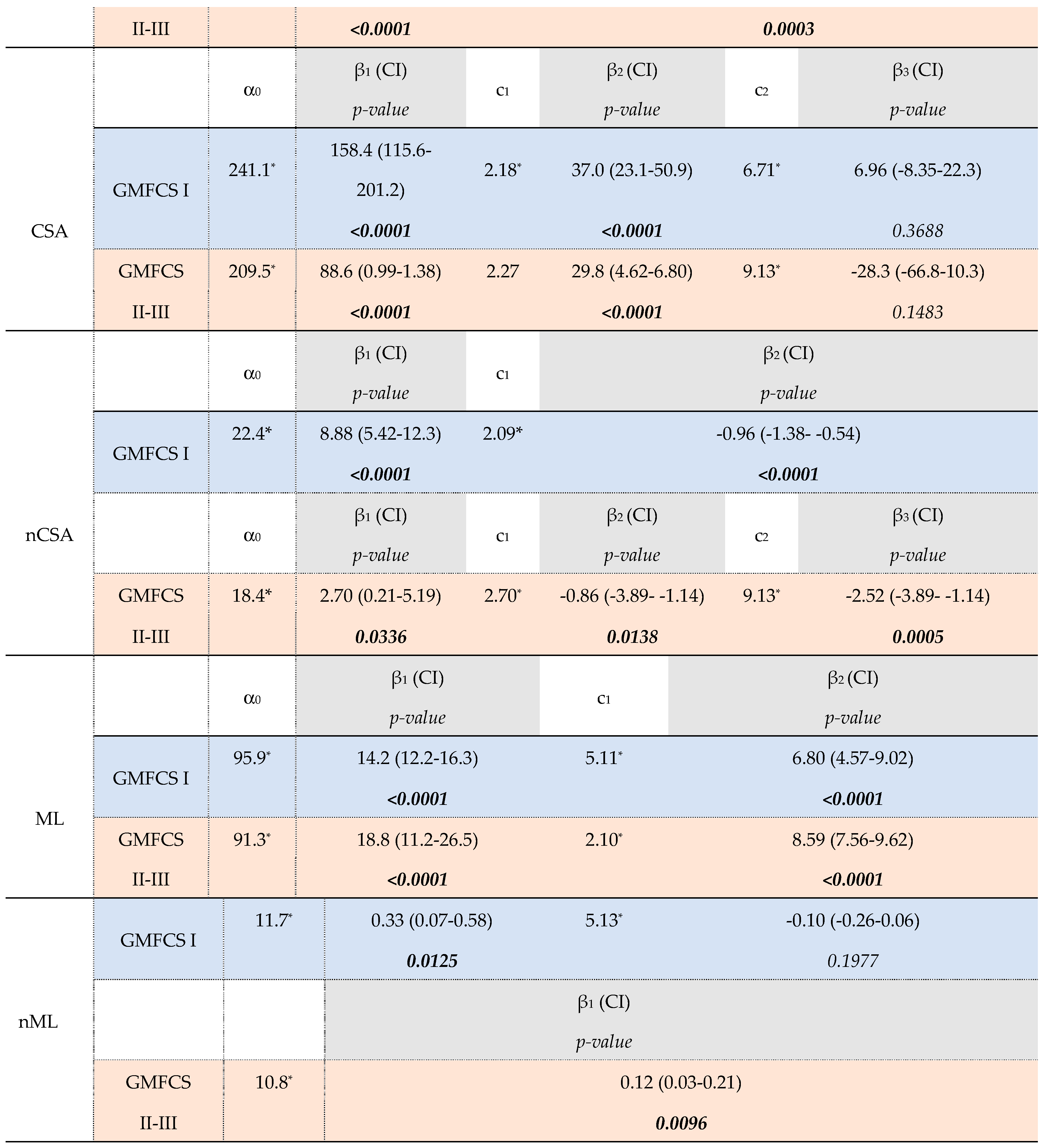
|
| Outcome | GMFCS I vs II- III | Δ | p-value |
| MV | β1, I vs. β1, II-III | 8.24 | 0.0003 |
| β2, I vs. β1, II-III | 1.16 | 0.0712 | |
| β 3, I vs. β2, II-III | 3.91 | 0.1069 | |
| c2, I vs c1, II-III | 1.31 | 0.0326 | |
| nMV | β1, I vs. β1, II-II | 0.55 | 0.0007 |
| β2, I vs. β2 II-III | 0.04 | 0.1710 | |
| β 3, I vs. β2, II-III | 0.05 | 0.1272 | |
| c1, I vs c1, II-III | 0.58 | 0.0040 | |
| α1, I vs α1, II-III | 0.32 | 0.0056 | |
| CSA | β1, I vs. β1, II-III | 69.8 | 0.0112 |
| β2, I vs. β2, II-III | 7.17 | 0.4328 | |
| β3, I vs. β3, II-III | 35.2 | 0.0915 | |
| c1, I vs c1, II-III | 0.09 | 0.3783 | |
| c2, I vs c2, II-III | 2.66 | 0.0033 | |
| nCSA | β1, I vs. β1, II-II | 6.18 | 0.0039 |
| β2, I vs. β2, II-III | -0.09 | 0.8168 | |
| β2, I vs β3, II-III | 1.56 | 0.0334 | |
| c1, I vs c1, II-III | 0.61 | 0.1152 | |
| α0, I vs α0, II-III | 3.97 | 0.0063 | |
| ML | β1, I vs. β1, II-II | -4.61 | 0.2482 |
| β2, I vs. β2, II-III | -1.79 | 0.1384 | |
| c1 vs c1 | -3.01 | <0.001 | |
| nML | β1, I vs. β1, II-II | 0.21 | 0.1311 |
| β2, I vs. β1, III-II | -0.22 | 0.0146 | |
| α0, I vs α0., II-III | 0.91 | 0.0024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





