1. Introduction
One of the factors that makes labor diagnosis difficult[
1] is that it must frequently take place in a:
“high pressured environment where conflicting pressures of workload, limited resources and emotional pressures add to the complexity of the judgement” [
2]. A wrong diagnosis can lead to labor mismanagement, as early accelerations and/or misdiagnoses of prolonged labor which can result in inappropriate monitoring and fetal distress [
3,
4]. Aside from that, timely diagnosis is likely to reduce the number of caesarian section [
5] as well as the number of unnecessary interventions during childbirth [
6,
7,
8].
However, in the current state of knowledge, there is little consensus regarding definitions of labor onset in the research literature [
9]. Currently, there is not precise clinical definition for the moment at which labor begins.
In 2005 we published “Diagnosis of Labor: a Prospective Study” [
1]. This multicentred prospective study assessed the predictability of the criteria for labor diagnosis most used in medical literature.
The study involved 423 pregnant women who presented to 2 Italian hospitals with uterine contractions (248 nulliparous patients total and 175 multiparous total) and were either admitted or advised to return home. The goal was to identify prospective criteria for labor diagnosis. The multivariant statistical analysis revealed four clinical criteria, that were significant predictors of labor onset:
Backache had instead a negative diagnostic value.
Regular uterine contractions, mucous plug loss, changes in intestinal habits, vomiting, pain relieved by walking, and changes in breathing pattern, did not have any diagnostic value.
We subsequently confirmed these results in a second prospective longitudinal cohort study, performed on a larger number of women [
10]. Although both studies were prospective, the methods used allowed only for a retrospective labor diagnosis, as in current clinical practice. To overcome this problem, we used metabolomic technology to analyse urine samples of patients in and out of labor, demonstrating that the metabolic patterns in the two groups are very different, despite the fact that clinical examination revealed no clear distinction. Without performing any obstetric examination, it is possible to clearly distinguish laboring patients from non-laboring patients by performing a metabolic examination of maternal urine (GC / MS and NMR approach) [
11]. In this study the list of discriminant urinary metabolites identified as significant during labor by H-NMR analysis allows us to discriminate in 100% of cases between patients in labor and patients not in labor. Some of the molecules that allow this discrimination, in decreasing order of importance are: glycine, lactic acid,3-hydroxybutyric acid, acetoacetic acid, acetone, and glucose. On the contrary and surprisingly, myoinositol revealed no discriminant results. Ultimately using a misclassification table, we were able to significantly predict a correct rate of laboring women in 100% of cases.
The metabolomics analysis evidenced clusters of metabolites involved in labor condition. Through the analysis of urine samples, collected before the onset and during labor, we were able to discriminate between laboring and non-laboring women, potentially offering the promise of a robust screening test to diagnose labor prospectively.
Nowadays one of the main problems in diagnosis is that there is little consensus in medical literature on the definitions of labor onset. A measurable definition of labor onset is therefore essential to avoid misdiagnosis and to identify deviations from normal in a timely manner [
9].We believe that this step is possible today, by making full use of the possibilities offered by the omics sciences.
In our first study [
1] despite focusing on labor diagnosis with only clinical instruments, obstetric examination, and history, we were unable to make a proper diagnosis of labor in 24% of cases. According to our studies, labor is characterized by a different maternal metabolic state. Why is it so difficult to prospectively diagnose labor in humans? To answer this question, we attempted an evolutionary interpretation of these data. The metabolic separation between a human female in labor and a non-laboring female is most likely archaic and predates time.
2. Analysis of Clinical Studies Considering the Evolutionary Framework
We reviewed the results of our studies on the diagnosis of labor and placed them in an evolutionary context to develop a hypothesis that explains why it is difficult to diagnose labor using clinical criteria, while the diagnosis becomes simple using complex metabolic criteria.
We have divided the criteria normally used to diagnose labor into items that can be easily hidden and not, from an outside observer (
Table 1 and
Table 2).
Concealment concerns the immediate external manifestation of the event; an element cannot be concealed when an observer or predator external to the event can notice, without refined investigations, the relatively incapacitated situation of the parturient, who is particularly vulnerable at that time.
Vomiting and changes in respiration are considered as clearly evident to an outside observer, as well as pain and suffering of the parturient, and are therefore classified as “evident criteria”, while variations of pain intensity, contractions frequency and dilation are instead among what we considered as “hidden” criteria.
In a primitive environment, and certainly in today's animal world as well, also the loss of the mucous plug (odor) can be classified as evident. As the mucus plug, the afterbirth as well is considered as an undeniable sign of delivery. Apart for the physical presence of the excreted placenta, the blood that comes with it and its smell can be easily detected by predators or other individuals passing nearby. Perhaps this is why many animals practice placentophagy. Of course this is not the only reason for placentophagy, and perhaps this is not even the most important reason. The placenta contains high levels of prostaglandins, which stimulate involution of the maternal uterus, and it also contains small amounts of oxytocin, which causes mammary myocells to contract to promote milk excretion. However, and more importantly, animals are also likely to practice placentophagy in order to leave no trace of childbirth behind, to protect newborns from predators [
12].
Concerning pain and thus the presence of contractile activity, we have considered them as evident from the outside, as well as the alleviation of pain through walking, as they cause changes in maternal behavior. However, variations in pain intensity over time may not be externally visible. Pain has in fact no valid and definitive units of measurement [
13], which makes it difficult for potential predators to understand the actual level of incapacitation of the mother, but also for those who assist the mother in our current setting to measure it, making visual or verbal analog scales, such as the Vas/Hutchison scale [
14], necessary.
Therefore, we believe that the reduction in the interval between uterine contractions and the increasing pain intensity are not externally perceptible. They are quantitative events that modify an already present reality.
Cervical distension and dilatation as well are certainly not visible, as they require gynecological evaluation to be determined.
Not surprisingly, the clinical criteria that resulted the most significant for diagnosis of labor in our study [
1] were: reduced interval between consecutive uterine contractions, pain of increasing intensity, and cervical distension and dilatation, aka all the “hidden” criteria. It only seems natural, according to the ancient modalities of birth, that the most important factors determining labor where those not readily accessible to outside observers and potential predators.
Childbirth, in fact, is typically a solitary event for nonhuman primates [
15]. The female who is in labor is extremely vulnerable, and this is why labor must be concealed or hidden. Over time, it is likely that only those elements that could be mystified, unseen by any outside predators, have been selected and passed the screening process.
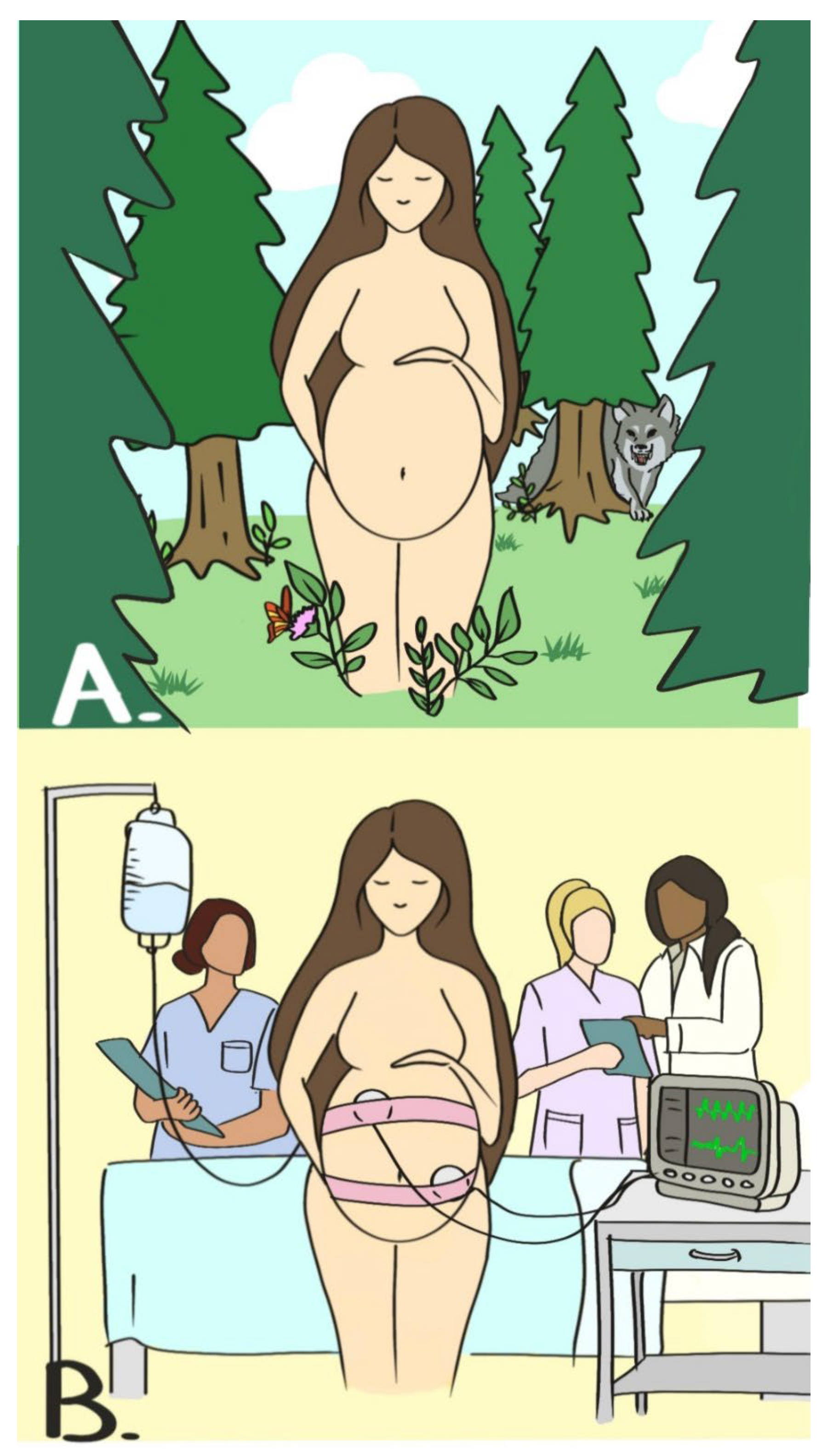
Thus, while childbirth, by its nature, requires secrecy, nowadays we give birth involving many people (
Figure 1). When did this start? When did women start asking for help? In other words, when was obstetrics born?
The ability to ask for help requires the development of certain skills, essentially those of verbal and nonverbal communication. Verbal skills are one of the features of intelligence most directly related (on a logarithmic scale) to increased telencephalic brain volume [
16]. Increased brain volume and bipedalism are probably the main factors that determined the radical difference between the different modalities of delivery in primates and humans. For the human fetus, there is always the need to perform rotational and flexion movements within the birth canal. In other words, the fetus must “negotiate” delivery, whereas in monkeys and apes this need is sporadic.
The remodeling of the female pelvis altered the birth canal to allow the birth of infants with larger brains than their predecessors. It was at this point that delivery assistance made a critical difference in mortality and morbidity for humans. Even today, obstructed labor is the leading cause of death in places where obstetric care is not readily available [
17,
18].
3. Labor Across Humans and Non-Human Primates
Comparisons between labor in other primates and humans throughout the years have revealed three basic differences or paradigms:
The mechanism of birth is always different, with the human infant's head and shoulders undergoing a series of rotational, bending, and deflection movements during labor.
The human infant is born through the pelvis, usually in the anterior occipito-frontal position (facing the mother);
In non-human primates, childbirth is usually a solitary event [
15].
This knowledge has been recently challenged through studies that proved that animals have in fact similar birth dynamics as humans. Firstly, the hypothesis of the fetus's head and shoulders negotiation for passage through the pelvis has been successfully defied [
20,
21]. Although in many cases (most of the childbirths observed so far) the fetuses of non-human primates do not appear undergoing a series of rotational, bending, and deflection movements during labor, in some cases this happens, showing that the difference is quantitative and not qualitative. It is likely that observation of more births in captivity and in the wild will enable us to understand that many other species besides humans must rotate in the pelvis [
22].
Moreover, it has been demonstrated that birth in the anterior occipito-fetal position is not a unique human prerogative [
21]. It was previously thought that, with the baby facing down, it was harder for the mother to hold the infant at the moment of childbirth, and this was supposedly the reason why women seek assistance from an external person, to grab and help the baby out when they couldn’t reach their child themselves. However, the fact that other species assume similar fetal positions suggests that it may not be a prerequisite for birth assistance.
Furthermore, human childbirth often occurs in forward-facing rotation [
23].
These two paradigms just described have been strongly questioned, even by their own authors [
24] due to a growing body of evidence demonstrating that the differences between human and other primate births are quantitative rather than qualitative in nature.
Regarding the third paradigm, it is unimportant in this context whether labor is manifested to other homo- or heterospecific individuals. The general rule is that primates give birth where and when members of the same group or predators are less likely to be present. Laboring females generally isolate themselves, often in trees, to seek protection from terrestrial predators [
25,
26]. In humans, instead, birth today almost always involves other people (
Figure 1). Unlike non-human primates, which seek solitude at the time of childbirth, human mothers actively seek assistance at birth. Human birth generally occurs in a social context, with help from other people [
27,
28]. All women, in almost all societies, seek assistance in childbirth from relatives, midwives or doctors. Trevathan call this “behavioral characteristic of seeking assistance at birth” [
27].
However, even this distinction no longer appears to be significant, and, more importantly, it is not unique, as there are exceptions to this rule, for example in the! Kung population, a hunter tribe from southern Africa, where women give birth alone in about half of the cases [
29].
Exception also the other way around were recorded, as in the wild white-headed langur (Trachypithecus leucocephalus) species, during a birth where a multiparous nonparturient female intervened in the delivery of an infant of another female by pulling it out of the pelvis and then holding and nursing it [
30]. The example reported by Pan Y. et al is not the only one in literature [
31]. In another case a non-parturient female black-and-white snub-nosed monkey (Rhinopithecus bieti) assisted the delivery of another female. In a birth reported in wild capped langurs (
Trachypithecus pileatus), the females of the group were seen to gather around the parturient and groom her during labor [
32]. Again, the differences are quantitative and not qualitative: for humans childbirth is
generally a social event.
At some point in human evolution, the advantages of childbirth care outweighed the disadvantages of close contact with other people such as stress caused by their presence during childbirth [
33] and infections, as the large blood loss that can occur during childbirth facilitates microorganisms’ transmission through the genital vascular system [
34].
“Obligate midwifery” then emerged as one of the most distinctive characteristics of the human species [
35,
36].
This is why a woman who gives birth alone arouses interest [
37]. So, although there may be rare exceptions, childbirth assistance is a phenomenon that has become almost universal in our species. The high mortality rates due to mechanical difficulties in childbirth contributed to this outcome; today, even in places where obstetric care is not widely available, obstructed labor is the leading cause of maternal death and morbidity [
17,
18]. One of the most important problems in modern obstetrics is that most births in the world take place without skilled assistance [
38].
In some states the case fatality rate for obstructed labour is 1.2% [
38,
39].
All of this has resulted in the first forms of obstetric assistance, though it is likely that assistance, which means getting your hands on childbirth, has contributed to a rise in infections, even fatal ones [
34].
Consider that in the first half of the nineteenth century in Vienna, Budapest, and Dublin, the percentage of maternal deaths (many of which were caused by puerperal sepsis) ranged from 0.39% to 10% [
40]. The benefit of skilled assistance and companionship during childbirth, on the other hand, is still recognized as one of the few certain factors that contribute to the success of a normal labor and delivery [
41,
42].
Although, as previously stated, this behavior is not unique to humans [
21] obstetrical challenges and the specific pattern of fetal emergence in humans may not be the origin of midwifery. The origin and function of birth care must be reconsidered in the context of biological and social evolution [
43].
4. Evolution of Human Language and Obstetric Care
The increase in cognitive abilities brought about by the telencephalon's expansion led to the development of refined forms of interhuman communication, most notably language, which appears to be a necessary condition for the emergence of obstetrical care; at some point, women began to ask for help, lest they die in loneliness in childbirth. Biological evolution took on the forms of cultural evolution. The cultural development that has resulted in our current social behavior took place, for the most part, within the last hundred thousand years, most likely because the small population that gave rise to all humans living today had achieved today's ability to communicate around that time [
44].
According to Falk [
45], it all began when the hominins started to walk upright, freeing their limbs to pick berries and fruit and then hunting. The female pelvis became narrower, and childbirth became more difficult. Human infants lost their ability to cling to a mother's chest and hair, who needed to use her arms to forage for food. As a result, the little ones were placed on the ground with increasing frequency, and Falk believes that this created a need to maintain constant vocal and emotional contact through verses and sound emissions. From these vocalizations the “motherese” developed, and from it a proto-language that gave rise to an increasingly sophisticated and rich form of communication. Even in the case of language as a form of communication, the differences are quantitative and not qualitative, as many other living beings use different forms of communication and the skills that enable speech appear early on the evolutionary ladder [
46]. Certainly, sudden or progressive physical changes have occurred in various stages and times of human evolution, such as the one described by Nishimura et al. [
47] that provides clear evidence that loss of vocal fold membranes, universal in nonhuman primates, resulted in a stable vocal source in humans, which had critical role in speech evolution.
In Falk’s hypothesis, at the basis of language and speech there are not cries of alarm and warning of danger, but the affective bond that, from an evolutionary perspective, places the female figure at the center of a fundamental phenomenon for our species: the development of human language, which over time has led to the emergence of, among other things, midwifery care [
45,
48,
49].
5. The Concept of Evolutionary Mismatch
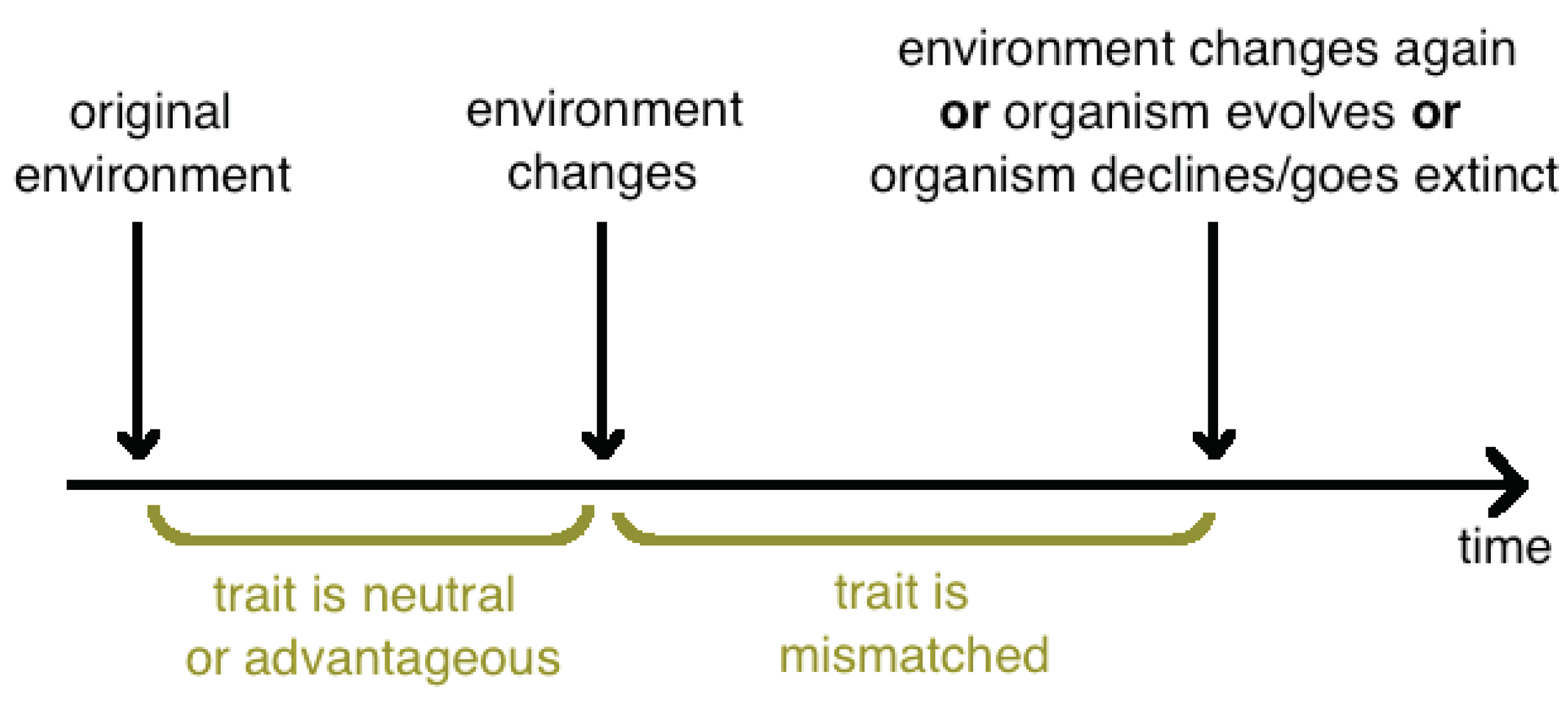
Evolutionary mismatch, or evolutionary trap, is a concept in evolutionary biology [
50] that refers to evolved traits that were once advantageous, but became maladaptive due to changes in the environment (
Figure 2).
A developmental mismatch occurs when the phenotype induced during development encounters a different environment post-development [
51]. Mismatch is an integral part of evolution in changing environments and is becoming increasingly common for all species living in human-altered environments. There are numerous examples of mismatches in the literature [
52]. Most species have evolved as niche constructors [
53] creating a constant environment through their activities and behaviors, all leading to a situation of equilibrium. Modern humans, on the other hand, constantly change their environment, rather than creating balance, they live in the constant instability that they themselves produce. In other words, humans, thanks to their cognitive and advanced learning abilities and manual dexterity, continuously change the environment in which they live, thus facilitating cultural evolution. This change is accelerated through the exponential increase in the advancement of technologies [
54], exposing contemporary humans to entirely new environments in the context of our evolutionary history. This dynamic has been beneficial for a long time, as long as our species lived in a hostile environment, almost totally devoid of technological capabilities, but it becomes very negative today, since our technological potential allows us to destroy the planet, through global warming [
55,
56], pollution [
57,
58,
59,
60], plastics [
61] or nuclear war [
62].
In case of diagnosis of labor, mismatches have occurred primarily due to cultural human evolution, and not only genetic evolution.
The modern human environment where childbirth takes place, is radically different from the environments we experienced as hunter-gatherers only a few hundred years ago.
In a hostile world, characterized by childbirth in solitude, hiding the signs and symptoms of labor was vital to survive together with the fetus, vice versa in the current environmental context, characterized by childbirth as a social phenomenon, that occurs mostly with assistance (specialized or non-specialized) of other human beings, these evolutionary traits (hiding the signs and symptoms of labor) are highly disadvantageous, since they do not allow a prompt and simple diagnosis, and therefore the implementation of a series of care attitudes that can help the woman in labor.
6. Conclusions
At some point in human evolution, the advantages of birth care outweighed the disadvantages, such as infections or stress resulting from close contact with other people, at a time when infections are easy to pass on, because of the risk of microorganisms entry by ascending route and of the great blood loss that can occur at birth, thus one of the most typical characteristics of the human species has emerged: “obligate midwifery”[
28]. Human childbirth can rightly be considered a social event. Although there may be rare exceptions, childbirth assistance is a phenomenon that has become universal in our species. When did this radical change begin? It is a question to this day unanswered, but omics sciences can help explain what happened so far back in time. Moreover, even from a strictly practical point of view, they will be able to fulfill what is called The Bio Revolution, namely: “a confluence of advances in biological sciences, decades in the making, with the accelerating development of computing, automation, and artificial intelligence, is fueling a new wave of innovation that could have significant impact on economies and societies, from health and agriculture to consumer goods and energy...”[
63].
Evolutionary mismatch is a situation that can only be addressed by behavioral adaptation, subsequent evolution, or other environmental change, and, in our case, appropriate use of metabolomic technologies. As we have seen in this review, the differences between humans and primates depend on the point of view and the quantitative over qualitative analysis that we pursue.
The diagnosis of labor is clinically very difficult, without the aid of metabolomics tools, as clearly demonstrated by the work of Ball, Jean A. and Marie Washbrook [
65] which reported that up to 30% of women admitted to labour wards in the UK were subsequently found not to be in labour.
Ina A. Stelzer et al. conducted an interesting longitudinal study in 63 women who went into labor spontaneously [
66]. More than 7000 plasma analyzes, and peripheral immune cell responses were analyzed using untargeted mass spectrometry, aptamer-based proteomic technology, and single-cell mass cytometry in serial blood samples collected during the last 100 days of pregnancy. The dataset was integrated into a multiomic model that predicted the time to spontaneous labor. The authors demonstrated that as the onset of labor approaches, a surge in steroid hormone metabolites and interleukin-1 receptor type 4 occurs, and this coincided with a switch from immune activation to regulation of inflammatory responses. Studies like this are very interesting, however predicting when labor will start, while useful, is very different from diagnosing labor when it has already started. Furthermore, the cost of integrated methods such as the one described is much higher than the possibility of using a simple urine stick derived from metabolomic analyses.
There is a great deal of literature demonstrating that the passage of the immune phenotype of the fetus-placental unit from predominantly tolerogenic to proinflammatory determines the onset of labor. At a certain moment of gestation, a cascade of events begins, characterized by the recruitment of maternal and fetal proinflammatory factors, immune cells of the uterine myometrium, the maternal cervix and the fetal chorioamniotic membranes. Although not all the mechanisms that determine the shift are well known, it is probable that the activation mechanism is determined by a placental clock [
67].
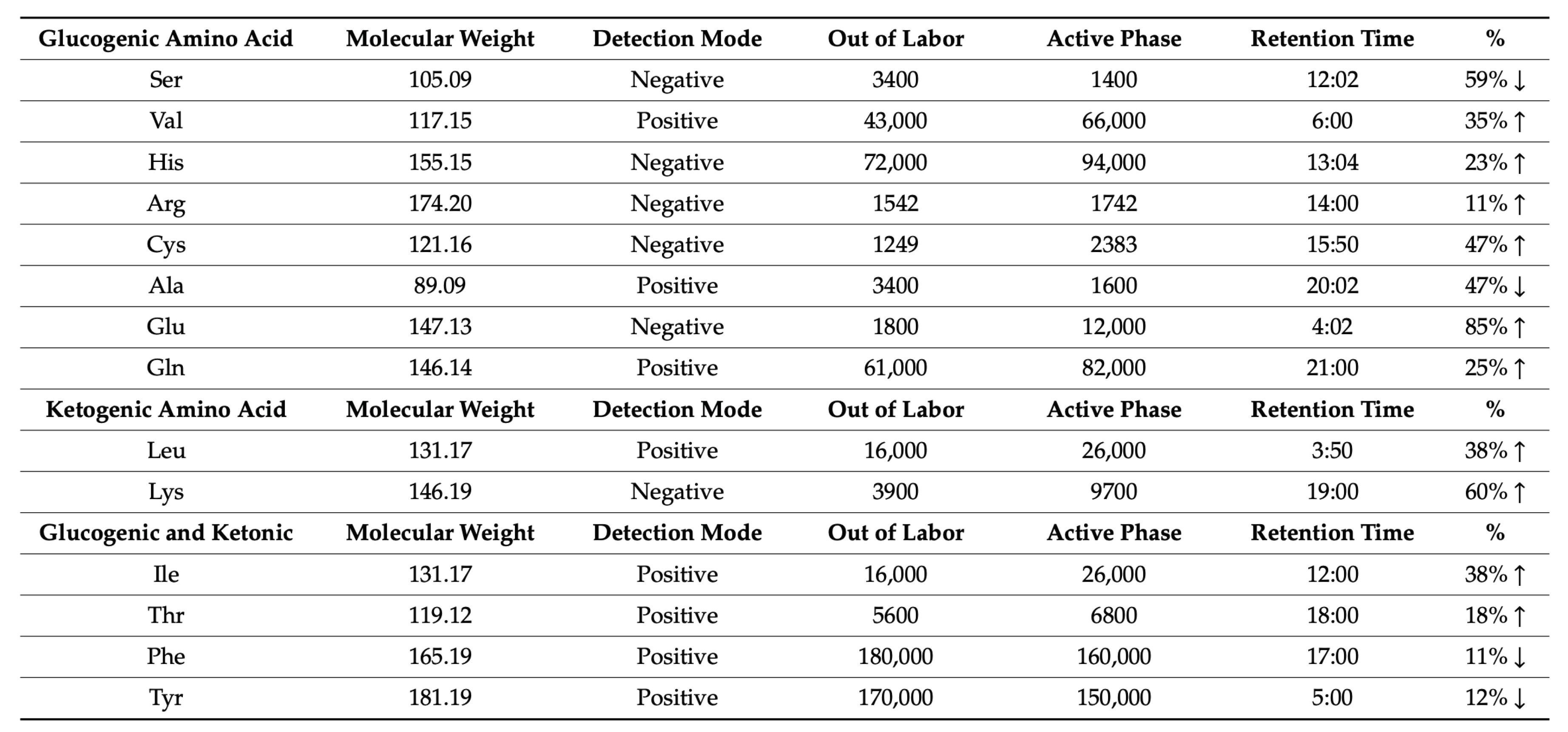
Metabolomics today is recognized as a powerful approach in a prenatal research context, since it can provide detailed information on women in and out of labor. In an observational, longitudinal, prospective cohort study of a total of 51 serial urine samples from 15 healthy pregnant women, Federica Gevi et al described the top 20 most discriminative metabolites contributing to the complete separation between laboring and non-laboring women [
68]. Urinary metabolites displaying the largest differences between laboring and non- laboring women belonged to steroid hormone, particularly conjugated estrogens and amino acids, and much of this difference is determined by the fetal contribution (
Table 3).
We must therefore use the technologies that we dispose of to accommodate the cultural changes that have had an impact on labor, since women’s bodies are still biologically wired, as other species in nature, to hid important clinical clues about their laboring status to external individuals. A device, such as a simple urine stick, should be developed to assess the biomolecules that result in 100% clear separation of the two populations of women in labor and out of labor. A simple POC test such as this would allow for better, less traumatic, and more effective management of human labor, and an actual cultural adaptation to the evolutionary mismatch. Ultimately, this paper demonstrates that metabolomics, in addition to being a great avenue for the future, also represents a method for uncovering the past.
Author Contributions
Conceptualization, A.R., AM methodology, A.R. original draft preparation, A.R. writing, review, and editing, A.R. and M.M., AM., DR. original drawings created by, M.M. visualization and supervision, A.R. project administration and funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in A. Ragusa, M. Mansur, A. Zanini, M. Musicco, L. Maccario, and G. Borsellino, “Diagnosis of labor: a prospective study.,” MedGenMed, vol. 7, no. 3, p. 61, Aug. 2005. at
https://www.medscape.com/viewarticle/509861.
Conflicts of Interest
The authors declare no conflict of interest.
References
- A. Ragusa, M. Mansur, A. Zanini, M. Musicco, L. Maccario, and G. Borsellino, “Diagnosis of labor: a prospective study.,” MedGenMed, vol. 7, no. 3, p. 61, Aug. 2005.
- A. H. Cheyne et al., “The development and testing of an algorithm for diagnosis of active labour in primiparous women,” Midwifery, vol. 24, no. 2, pp. 199–213, 2008. [CrossRef]
- K. O’Driscoll and D. Meagher, “Active Managment of Labour,” London: Saunders, 1980.
- S. Arukulmaran and S. Chua, “Augmentation in labour,” in Contributions to Obstetrics & Gynaecology, Vol. 3, S. Ratnam, S. Ng, D. Sen, and S. Arulkumaran, Eds. Longmans, Singapore: Churchill Livingstone, 1994.
- N. Saunders and H. Spiby, “Oxytocin in active-phase abnormalities of labor: a randomized study.,” Obstetrics and gynecology, vol. 76, no. 3 Pt 1. United States, p. 475, Sep. 1990.
- H. Cammu and E. Van Eeckhout, “A randomised controlled trial of early versus delayed use of amniotomy and oxytocin infusion in nulliparous labour.,” Br. J. Obstet. Gynaecol., vol. 103, no. 4, pp. 313–318, Apr. 1996. [CrossRef]
- A. Svelato, A. Ragusa, and P. Manfredi, “General methods for measuring and comparing medical interventions in childbirth: a framework,” BMC Pregnancy Childbirth, vol. 20, no. 1, p. 279, 2020. [CrossRef]
- A. Ragusa, S. Gizzo, M. Noventa, E. Ferrazzi, S. Deiana, and A. Svelato, “Prevention of primary caesarean delivery: comprehensive management of dystocia in nulliparous patients at term,” Arch. Gynecol. Obstet., vol. 294, 2016. [CrossRef]
- G. E. Hanley et al., “Diagnosing onset of labor: a systematic review of definitions in the research literature.,” BMC Pregnancy Childbirth, vol. 16, p. 71, Apr. 2016. [CrossRef]
- G. Loi, A. Meloni, G. Melis, S. Deiana, E. Ferrazzi, and A. Ragusa, “Diagnosis of labor: the II prospective clinical study,” in BGOG Volume 119, Issue Supplement s1, p. 52.
- P. Caboni et al., “Urinary metabolomics of pregnant women at term: a combined GC/MS and NMR approach.,” J. Matern. neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet., vol. 27 Suppl 2, pp. 4–12, Oct. 2014. [CrossRef]
- .
- M. B. Kristal, “Placentophagia: a biobehavioral enigma (or De gustibus non disputandum est).,” Neurosci. Biobehav. Rev., vol. 4, no. 2, pp. 141–150, 1980. [CrossRef]
- K. S. Kaiser, D. B. McGuire, T. J. Keay, and M. E. Haisfield-Wolfe, “Methodological challenges in conducting instrumentation research in non-communicative palliative care patients.,” Appl. Nurs. Res., vol. 51, p. 151199, Feb. 2020. [CrossRef]
- O. Karcioglu, H. Topacoglu, O. Dikme, and O. Dikme, “A systematic review of the pain scales in adults: Which to use?,” Am. J. Emerg. Med., vol. 36, no. 4, pp. 707–714, Apr. 2018. [CrossRef]
- A. JOLLY, “Primate birth hour,” Int. Zoo Yearb., vol. 13, no. 1, pp. 391–397, Jan. 1973. [CrossRef]
- S. F. Witelson, H. Beresh, and D. L. Kigar, “Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors.,” Brain, vol. 129, no. Pt 2, pp. 386–398, Feb. 2006. [CrossRef]
- Ranjana and A. Sinha, “Incidence, causes and feto-maternal outcomes of obstructed labour in a tertiary health care centre,” Int. J. Reprod. Contraception, Obstet. Gynecol., vol. 6, no. 7, p. 2817, 2017. [CrossRef]
- N. Rizwan and A. Mughal, “Frequency of maternal morbidity in women with obstructed labor: A study at Liaquat University Hospital Hyderabad Sindh,” Appl. Med. Res., vol. 4, no. 1, pp. 1–4, 2018, [Online]. Available: www.ejmamr.com.
- C. S. McClain, “Human birth: An evolutionary perspective,” Am. J. Phys. Anthropol., vol. 74, no. 4, pp. 544–545, Dec. 1987. [CrossRef]
- M. K. Stoller, “The obstetric pelvis and mechanism of labor in nonhuman primates (Ph. D. thesis) University of Chicago.” Chicago, 1995.
- S. Hirata, K. Fuwa, K. Sugama, K. Kusunoki, and H. Takeshita, “Mechanism of birth in chimpanzees: humans are not unique among primates,” Biol. Lett., vol. 7, no. 5, pp. 686–688, 2011. [CrossRef]
- R. Martin, How we do it: The evolution and future of human reproduction. Hachette UK, 2013.
- [A. Svelato et al., “Occiput-spine relationship: shoulders are more important than head,” Eur. Rev. Med. Pharmacol. Sci., vol. 2017, pp. 1178–1183, Apr. 2017.
- W. Trevathan, “Primate pelvic anatomy and implications for birth,” Philos. Trans. R. Soc. B Biol. Sci., vol. 370, no. 1663, p. 20140065, 2015. [CrossRef]
- C. J. Allan, P. J. Holst, and G. N. Hinch, “Behaviour of parturient Australian bush goats. I. Doe behaviour and kid vigour,” Appl. Anim. Behav. Sci., vol. 32, no. 1, pp. 55–64, 1991. [CrossRef]
- L. M. Lidfors, D. Moran, J. Jung, P. Jensen, and H. Castren, “Behaviour at calving and choice of calving place in cattle kept in different environments,” Appl. Anim. Behav. Sci., vol. 42, no. 1, pp. 11–28, 1994. [CrossRef]
- W. R. Trevathan, “Fetal emergence patterns in evolutionary perspective,” Am. Anthropol., vol. 90, no. 3, pp. 674–681, 1988. [CrossRef]
- K. R. Rosenberg, “The evolution of modern human childbirth,” Am. J. Phys. Anthropol., vol. 35, no. S15, pp. 89–124, 1992. [CrossRef]
- M. Konner and M. Shostak, “Timing and management of birth among the! Kung: Biocultural interaction in reproductive adaptation,” Cult. Anthropol., vol. 2, no. 1, pp. 11–28, 1987. [CrossRef]
- W. Pan et al., “Birth intervention and non-maternal infant-handling during parturition in a nonhuman primate,” Primates, vol. 55, no. 4, pp. 483–488, 2014. [CrossRef]
- W. Ding, L. Yang, and W. Xiao, “Daytime birth and parturition assistant behavior in wild black-and-white snub-nosed monkeys (Rhinopithecus bieti) Yunnan, China,” Behav. Processes, vol. 94, pp. 5–8, 2013. [CrossRef]
- A. Kumar, G. S. Solanki, and B. K. Sharma, “Observations on parturition and allomothering in wild capped langur (Trachypithecus pileatus),” Primates, vol. 46, no. 3, pp. 215–217, 2005. [CrossRef]
- F. Kazemi, S. Z. Masoumi, F. Soltani, K. Oshvandi, S. Ghelichkhani, and Z. Niazy, “Postpartum women’s perception of stressors in the delivery ward: a qualitative study,” BMC Res. Notes, vol. 13, no. 1, p. 335, 2020. [CrossRef]
- E. Contro and E. Jauniaux, “Puerperal sepsis: what has changed since Semmelweis’s time,” BJOG An Int. J. Obstet. Gynaecol., vol. 124, no. 6, p. 936, May 2017. [CrossRef]
- K. Rosenberg and W. Trevathan, “Birth, obstetrics and human evolution,” BJOG An Int. J. Obstet. Gynaecol., vol. 109, no. 11, pp. 1199–1206, 2002. [CrossRef]
- W. R. Trevathan, Human birth: An evolutionary perspective. Routledge, 2017. [CrossRef]
- M. Shostak, Nisa: The Life and Words of a !Kung Woman. Cambridge, Massachusetts: Harvard University Press, 1981, 1981.
- M. Carlough and M. McCall, “Skilled birth attendance: what does it mean and how can it be measured? A clinical skills assessment of maternal and child health workers in Nepal,” Int. J. Gynecol. Obstet., vol. 89, no. 2, pp. 200–208, 2005. [CrossRef]
- J. K. Kabakyenga, P.-O. Östergren, E. Turyakira, P. K. Mukasa, and K. O. Pettersson, “Individual and health facility factors and the risk for obstructed labour and its adverse outcomes in south-western Uganda,” BMC Pregnancy Childbirth, vol. 11, no. 1, pp. 1–10, 2011. [CrossRef]
- T. D. Noakes, J. Borresen, T. Hew-Butler, M. I. Lambert, and E. Jordaan, “Semmelweis and the aetiology of puerperal sepsis 160 years on: an historical review,” Epidemiol. Infect., vol. 136, no. 1, pp. 1–9, 2008. [CrossRef]
- M. A. Bohren, G. J. Hofmeyr, C. Sakala, R. K. Fukuzawa, and A. Cuthbert, “Continuous support for women during childbirth.,” Cochrane database Syst. Rev., vol. 7, no. 7, p. CD003766, Jul. 2017. [CrossRef]
- B. Chalmers and W. Wolman, “Social support in labor-a selective review,” J. Psychosom. Obstet. Gynecol., vol. 14, no. 1, pp. 1–15, 1993. [CrossRef]
- BBC Earth, The Monkey that Became a Midwife. “http://www.bbc.com/earth/story/20141006-the-monkey-that-became-a-midwife?ocid=ww.social.link.twitter.”.
- L. L. Cavalli-Sforza, Genes, Peoples, and Languages. Penguin Books Ltd, 2001.
- D. Falk et al., “Early hominid brain evolution: a new look at old endocasts,” J. Hum. Evol., vol. 38, no. 5, pp. 695–717, 2000. [CrossRef]
- D. Z. Narayanan, D. Y. Takahashi, L. M. Kelly, S. I. Hlavaty, J. Huang, and A. A. Ghazanfar, “Prenatal development of neonatal vocalizations,” Elife, vol. 11, p. e78485, 2022. [CrossRef]
- T. Nishimura et al., “Evolutionary loss of complexity in human vocal anatomy as an adaptation for speech,” Science (80-. )., vol. 377, no. 6607, pp. 760–763, 2022. [CrossRef]
- D. Falk, “Prelinguistic evolution in early hominins: Whence motherese?,” Behav. Brain Sci., vol. 27, no. 4, pp. 491–503, 2004. [CrossRef]
- I. Davidson et al., “The archaeology of perception: traces of depiction and language [and comments and reply],” Curr. Anthropol., vol. 30, no. 2, pp. 125–155, 1989. [CrossRef]
- E. Lloyd, D. S. Wilson, and E. Sober, “Evolutionary mismatch and what to do about it: A basic tutorial,” Evol. Appl., pp. 2–4, 2011.
- P. D. Gluckman, M. A. Hanson, and F. M. Low, “Evolutionary and developmental mismatches are consequences of adaptive developmental plasticity in humans and have implications for later disease risk,” Philos. Trans. R. Soc. B, vol. 374, no. 1770, p. 20180109, 2019. [CrossRef]
- K. J. Winkler, M. C. Dade, and J. T. Rieb, “Mismatches in the ecosystem services literature—a review of spatial, temporal, and functional-conceptual mismatches,” Curr. Landsc. Ecol. Reports, vol. 6, no. 2, pp. 23–34, 2021. [CrossRef]
- F. Odling-Smee, K. Laland, and M. Feldman, Niche construction: The neglected process in evolution. Princeton (New Jersey): Princeton University Press, 2003.
- R. W. Fogel, The Escape from Hunger and Premature Death, 1700–2100: Europe, America, and the Third World. Cambridge: Cambridge University Press, 2004. [CrossRef]
- S. Li et al., “Exploring associations of maternal exposure to ambient temperature with duration of gestation and birth weight: A prospective study,” BMC Pregnancy Childbirth, vol. 18, no. 1, pp. 1–14, 2018. [CrossRef]
- S. M. Holm and J. Balmes, “No fire without smoke (particles).,” Elife, vol. 10, Nov. 2021. [CrossRef]
- R. El Morabet, S. Mouak, R. A. Khan, A. A. El Ouadrhiri, and M. Aneflouss, “Effects of Outdoor Air Pollution on Human Health in Kenitra, Morocco,” in New Prospects in Environmental Geosciences and Hydrogeosciences, 2022, pp. 251–253. [CrossRef]
- V. Arroyo, J. Díaz, C. Ortiz, R. Carmona, M. Sáez, and C. Linares, “Short term effect of air pollution, noise and heat waves on preterm births in Madrid (Spain).,” Environ. Res., vol. 145, pp. 162–168, Feb. 2016. [CrossRef]
- B. Bekkar, S. Pacheco, R. Basu, and N. DeNicola, “Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review.,” JAMA Netw. open, vol. 3, no. 6, p. e208243, Jun. 2020. [CrossRef]
- D. A. Savitz et al., “Ambient fine particulate matter, nitrogen dioxide, and term birth weight in New York, New York.,” Am. J. Epidemiol., vol. 179, no. 4, pp. 457–466, Feb. 2014. [CrossRef]
- GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection, “Sources, fate and effects of microplastics in the marine environment: part 2 of a global assessment. (IMO, FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP). In: Kershaw, P.J. (Ed.), Rep. Stud. GESAMP No. 90 (96 pp).,” Reports Stud. GESAMP, No. 93, 96 p., vol. 93, 2016.
- F. Boulton and T. Dunn, “Nuclear war and public health: preparedness, protection and the case for prevention,” J. Public Health (Bangkok)., vol. 42, no. 3, pp. e316–e322, 2020. [CrossRef]
- M. Chui, M. Evers, J. Manyika, A. Zheng, and T. Nisbet, “The Bio Revolution,” McKinsey Glob. Inst., no. May, p. 200, 2020, [Online]. Available: https://www.mckinsey.com/mgi/overview.
- Ball, Jean A. and Marie Washbrook. “Birthrate plus : a framework for workforce planning and decision making for midwifery services.” (1996). https://www.semanticscholar.org/author/M.-Washbrook/46351260.
- Ina A. Stelzer1,†, Mohammad S. Ghaemi1,2,†, Xiaoyuan Han1,3,†, Kazuo Ando1,†, Julien J. Hédou1,†, Dorien Feyaerts1, Laura S. Peterson4, Kristen K. Rumer1, Eileen S. Tsai1, Edward A. Ganio1, Dyani K. Gaudillière5, Amy S. Tsai1, Benjamin Choisy1, Lea P. Gaigne1, Franck Verdonk1, Danielle Jacobsen1, Sonia Gavasso1,6, Gavin M. Traber7, Mathew Ellenberger7, Natalie Stanley1,8, Martin Becker1,8, Anthony Culos1,8, Ramin Fallahzadeh1,8, Ronald J. Wong4, Gary L. Darmstadt9, Maurice L. Druzin10, Virginia D. Winn11, Ronald S. Gibbs10, Xuefeng B. Ling12, Karl Sylvester12, Brendan Carvalho1, Michael P. Snyder7, Gary M. Shaw4, David K. Stevenson4, Kévin Contrepois7, Martin S. Angst1,‡, Nima Aghaeepour1,4,8,‡, Brice Gaudillière1,4,*,‡. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci Transl Med. 2021 May 05; 13(592). [CrossRef]
- Peterson, L.S., Stelzer, I.A., Tsai, A.S. et al. Multiomic immune clockworks of pregnancy. Semin Immunopathol 42, 397–412 (2020). https://doi.org/10.1007/s00281-019-00772-1. [CrossRef]
- Federica Gevi 1,†, Alessandra Meloni 2,†, Rossella Mereu 2, Veronica Lelli 1, Antonella Chiodo 2 , Antonio Ragusa 3 and Anna Maria Timperio 1,* . Urine Metabolome during Parturition. Metabolites 2020, 10, 290. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).