Submitted:
09 January 2023
Posted:
16 January 2023
You are already at the latest version
Abstract
Keywords:
1. What is Diffuse Optical Tomography
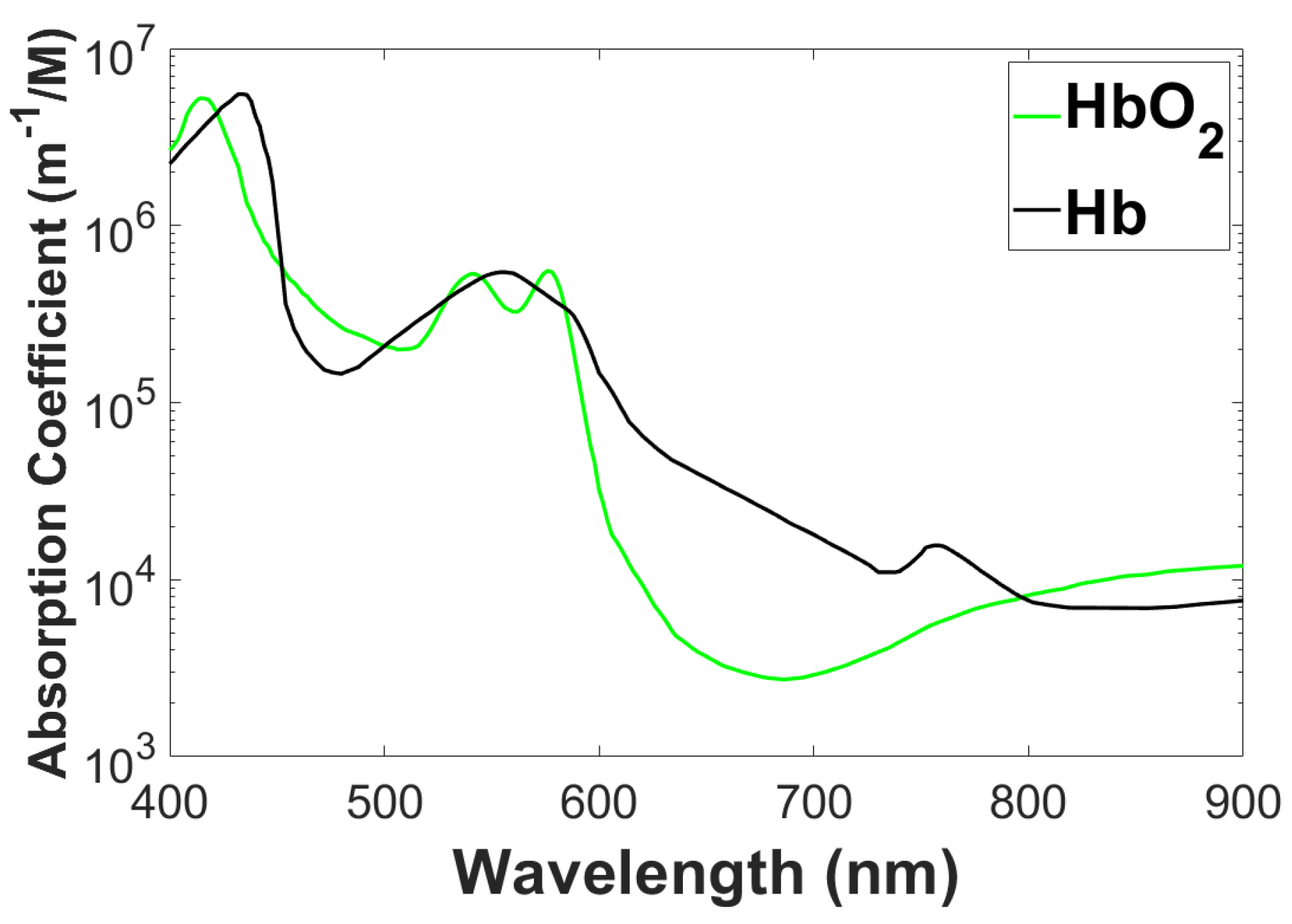
1.1. Application of DOT
- Breast cancer imaging: X-ray mammography can detect breast cancer. To improve the assessment and characterization of breast tumors, a wide range of other techniques such as ultrasound, Electrical Impedance Tomography (EIT), and Magnetic Resonance Imaging (MRI) are being used. Positron Emission Tomography (PET) and MRI are becoming more popular because they provide fundamentally different information than traditional structural pictures. It gives us direct access to physiological data like amount of blood, metabolic state, flow of blood, and oxygen level. Tumor angiogenesis alters these tissue characteristics, which are also used to track a tumor’s response to therapy. These functional parameters can be imaged by DOT. Because tumors are more vascularized than surrounding tissue, they absorb light differently. Through spectroscopic variation in , the relative concentration of oxygenated hemoglobin can be determined, and thus the oxygen demand-supply ratio can be determined. Furthermore, it can be used to differentiate tumors from background tissue, malignant tumors from non-malignant tumors, and tumors with varying levels of activity (degrees of malignancy).
- Brain function imaging: A DOT assessment of brain function complements positron emission tomography (PET), functional MRI (fMRI), electroencephalogram (EEG), and magnetoencephalography (MEG). PET images changes in metabolic activity but has a low temporal and spatial resolution. fMRI provide high spatial and temporal resolution images of blood flow and deoxy-hemoglobin concentration, but cannot measure oxyhemoglobin level simultaneously. EEG as well as MEG can monitor electrical activity of the brain with much higher time resolution (50 to 1 kHz), but pin pointing sources of these electrical and magnetic fields is difficult and resolution in space is not upto as expectation compared with fMRI. While its spatial resolution is inferior to that of fMRI, the DOT, when used in combination with fMRI, can simultaneously measure oxy- and deoxyhemoglobin concentrations and blood volume. Combining light-based imaging with fMRI and MEG/EEG could provide result in a complete picture that is more useful than any of the parts alone.
- Stroke: It may be possible to detect ischemic strokes and hemorrhagic strokes more quickly and accurately using DOT, which is essential before applying neuroprotective drugs as it can effectively treat stroke patients in case of ischemic strokes, but can lead to fatality in case of hemorrhagic strokes. It is also possible to monitor the progression of a stroke and treatment response using DOT at the bedside.
- Monitoring Brain Trauma and Surgical Intervention: Detecting a brain hemorrhage early can greatly improve a patient’s recovery and long-term effects if the hemorrhage occurs because of brain injury. Current screening methods include cognitive testing and invasive monitoring (e.g., measurement of cranial pressure). A continuous DOT monitor at the bedside could provide continuous monitoring on bleeding site and spreading, which is an advantage over these techniques. Collateral damage can also be minimized through monitoring during brain surgery. The use of an EEG while performing a surgery could disrupt the critical surgical functions, but the placement of electrodes is a time-consuming and painful process. There is also the option of using an fMRI, but a special room with a costly magnet is required for this. An inexpensive alternative could be the DOT that can provide optical imaging on the surface of the body.
2. Theoretical Basis of Diffuse Optical Tomography
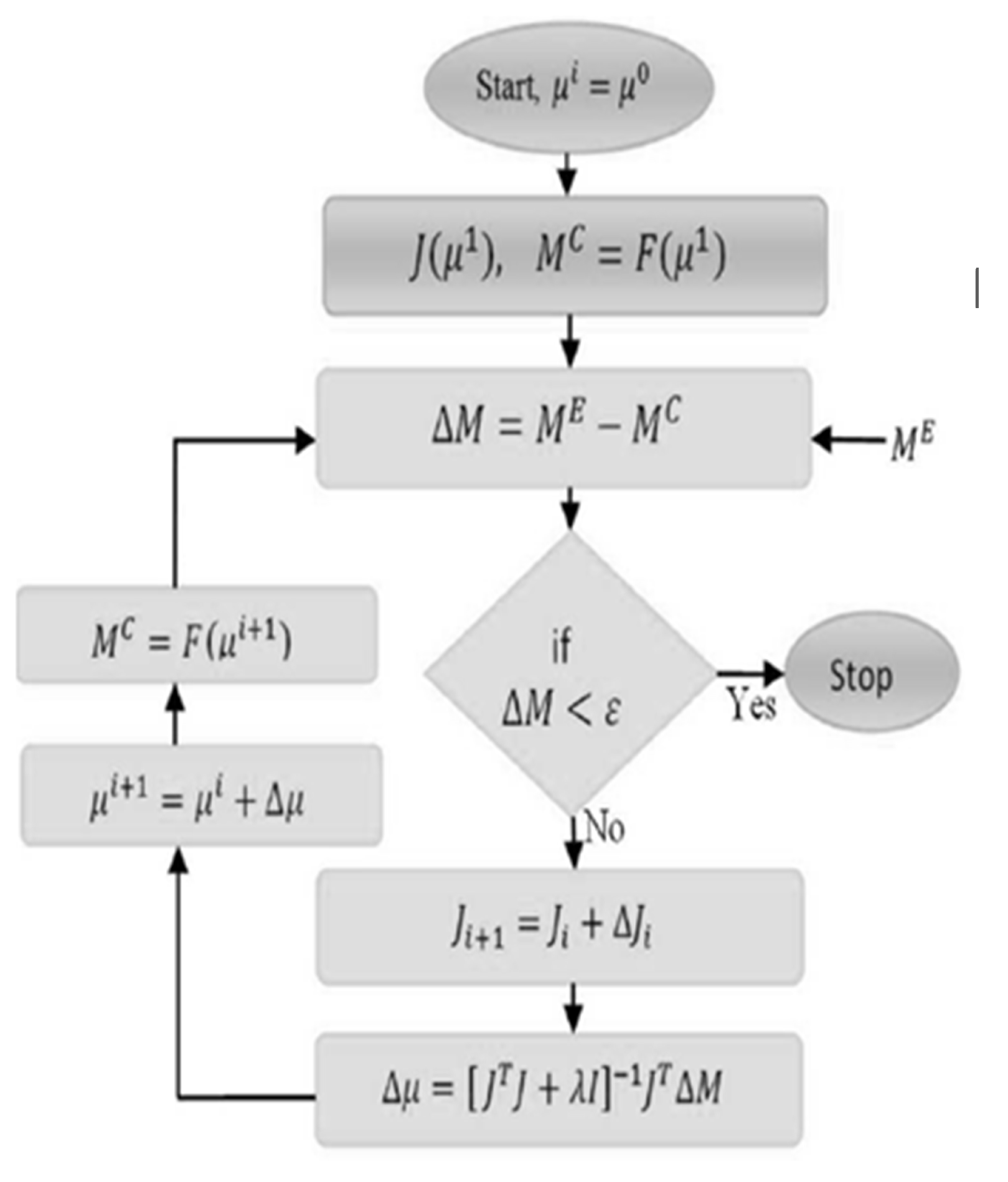
2.1. Photon Diffusion Equation
3. Difficulties in DOT Imaging
- The scattering nature of photons traveling through tissue makes DOT an attractive tool for the noninvasive imaging of diseases. The strong scattering of light by biological tissue leads to poor depth localization in DOT due to the attenuation of detection sensitivity exponentially with depth.
- The tissue is like a turbid property with heavily scattering property. Light travels through the tissue in a complicated zigzag path. As a result, strength attenuated. this renders the relation between the output photon density and the optical properties dependent on stochastically defined paths.
- In the imaging for frequency space, although the amplitude changes during modulation at Mega Hertz, the wavelength of DPWD (Diffuse Photon Density wave), which is owing to the intensity modulated illumination, is of the order of a few cm, much greater than the typical size of inhomogeneities. This is the fundamental reason for poor resolution in images from DOT.
- When one illuminates the turbid object with a short pulse, the ballistic photons are very few, or none. If ballistic photons are of sufficient strength reconstruction from such photons can give better-resolved images.
- The background properties being not known in advance leads to difficulties in measuring and interpreting measurements for inverse reconstructions.
- As a result of the quantum nature of noise, modeling it is a challenging task. This is because the sources of noise include both thermal noise in the amplification unit and the noise generated by the shots due to the quantum source nature.
- Absorption/scattering coefficients and field amplitude and phase are nonlinearly related. Due to these considerations, either a linearized approximation like Born or Rytov must be used or a nonlinear forward model must be used to reduce the numerical burden.
- Depending on the geometry and physical conditions, light may propagate in greatly different ways, for example through significantly scattered brain tissue that is covered by slightly scattering cerebrospinal fluid. In order for dealing with such inclusions the DE is inadequate as a model for light transport. One should rely on the RTE, which also takes into account the angles of scattering.
- The light interection of tissue are characterized by two parameters. Simultaneous reconstruction of both parameters complicates and may induce cross-talk between the images.
- Ill-posedness may also arise from the fact that very small changes in optical parameters can give rise to large changes in measurement or vice versa. Thus inverse solutions must take care to see that the effect of noise, which gives rise to large swings in reconstruction, is properly accounted for.
- In addition, one might estimate the absorption or scattering coefficient at many locations in space, several times of amplitude more than the taken datapoints. This is an ill-posed problem. This also becomes another source of non-uniqueness of solutions.
3.1. Progress and Future Directions
4. Discussion and Conclusion
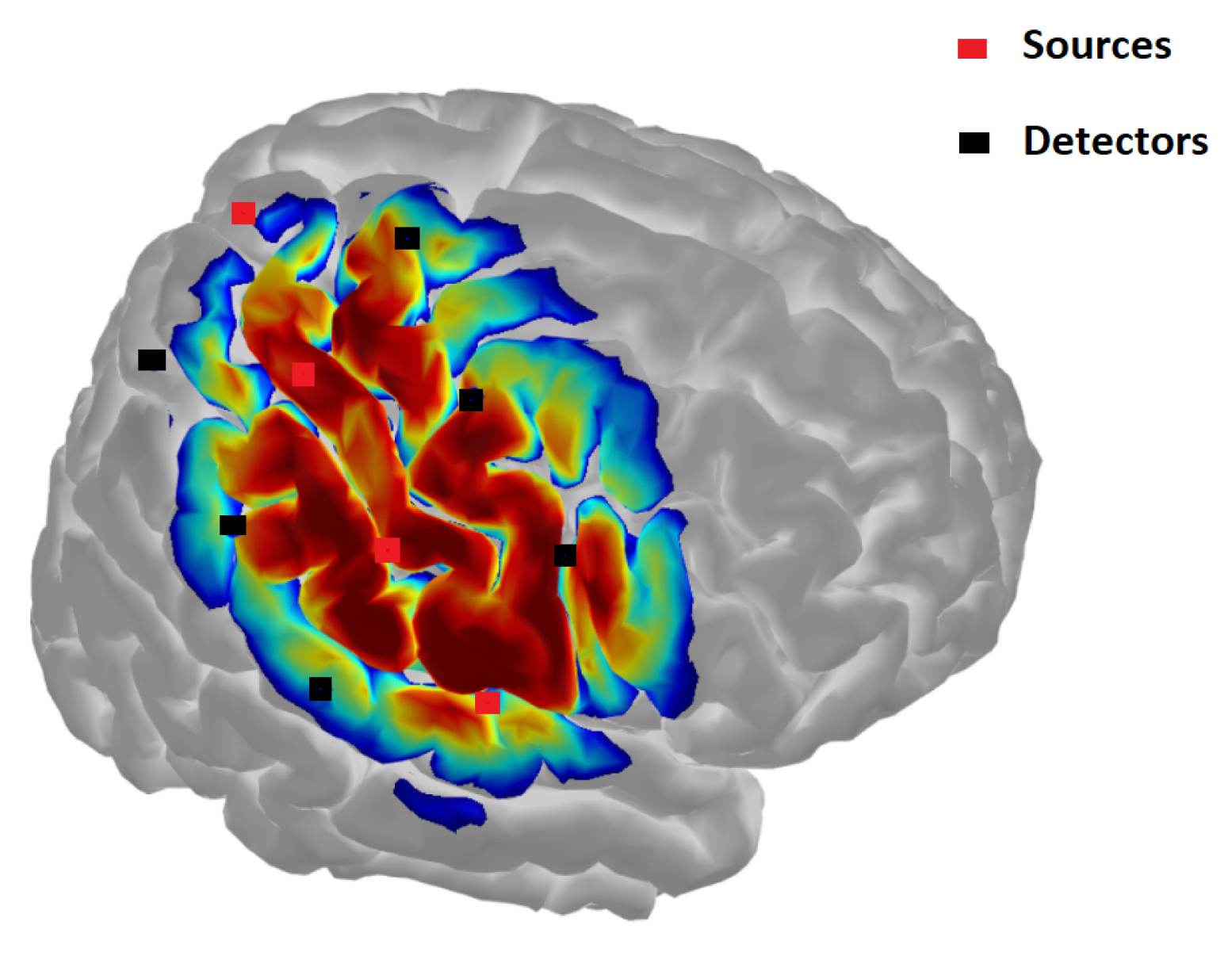
References
- Arridge, S.R. Optical tomography in medical imaging. Inverse Problems 1999, 15, R41. [Google Scholar] [CrossRef]
- Gibson, A.P.; Hebden, J.C.; Arridge, S.R. Recent advances in diffuse optical imaging. Physics in Medicine and Biology 2005, 50, R1. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.J. Design and development of a functional diffuse optical tomography probe for real-time 3D imaging of tissue. SPIE 2021, 11639, 213–218. [Google Scholar]
- Arridge, S.R.; Hebden, J.C. Optical imaging in medicine: II. Modelling and reconstruction. Physics in Medicine and Biology 1997, 42, 841. [Google Scholar] [CrossRef]
- Poorna, R.; Kanhirodan, R.; Saikia, M.J. Square-waves for frequency multiplexing for fully parallel 3D diffuse optical tomography measurement. SPIE 2021, 11639, 219–226. [Google Scholar] [CrossRef]
- Saikia, M.J. A spectroscopic diffuse optical tomography system for the continuous 3D functional imaging of tissue -a phantom study. IEEE Transactions on Instrumentation and Measurement 2021, 1. [Google Scholar] [CrossRef]
- Saikia, M.J.; Kanhirodan, R. Development of handheld near-infrared spectroscopic medical imaging system. Optical Society of America, 2019, p. DS1A.6.
- Chance, B.; Leigh, J.S.; Miyake, H.; Smith, D.S.; Nioka, S.; Greenfeld, R.; Finander, M.; Kaufmann, K.; Levy, W.; Young, M. Comparison of time-resolved and -unresolved measurements of deoxyhemoglobin in brain. Proceedings of the National Academy of Sciences of the United States of America 1988, 85, 4971–4975. [Google Scholar] [CrossRef]
- Knüttel, A.; Schmitt, J.M.; Knutson, J.R. Spatial localization of absorbing bodies by interfering diffusive photon-density waves. Applied optics 1993, 32, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; O’Leary, M.A.; Chance, B.; Yodh, A.G. Scattering of diffuse photon density waves by spherical inhomogeneities within turbid media: analytic solution and applications. Proceedings of the National Academy of Sciences of the United States of America 1994, 91, 4887–4891. [Google Scholar] [CrossRef]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties. Appl. Opt. 1989, 28, 2331–2336. [Google Scholar] [CrossRef]
- Patterson, M.S.; Moulton, J.D.; Wilson, B.C.; Berndt, K.W.; Lakowicz, J.R. Frequency-domain reflectance for the determination of the scattering and absorption properties of tissue. Appl. Opt. 1991, 30, 4474–4476. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Nioka, S.; Kent, J.; McCully, K.; Fountain, M.; Greenfeld, R.; Holtom, G. Time-resolved spectroscopy of hemoglobin and myoglobin in resting and ischemic muscle. Analytical biochemistry 1988, 174, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Whitlock, T.L.; Cope, M.; Delpy, D.T. Multiwavelength, wideband, intensity-modulated optical spectrometer for near-infrared spectroscopy and imaging. SPIE 1993, 1888, 248–257. [Google Scholar] [CrossRef]
- Ferrari, M.; Wei, Q.; Carraresi, L.; Blasi, R.A.D.; Zaccanti, G. Time-resolved spectroscopy of the human forearm. Journal of photochemistry and photobiology. B, Biology 1992, 16, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Wilson, D.A.; Hanley, D.F.; Hartmann, J.F.; Rogers, M.C.; Traystman, R.J. Noninvasive determination of hemoglobin saturation in dogs by derivative near-infrared spectroscopy. The American journal of physiology 1989, 256, H1493–9. [Google Scholar] [CrossRef] [PubMed]
- Sevick, E.M.; Chance, B.; Leigh, J.; Nioka, S.; Maris, M. Quantitation of time- and frequency-resolved optical spectra for the determination of tissue oxygenation. Analytical biochemistry 1991, 195, 330–351. [Google Scholar] [CrossRef] [PubMed]
- Svaasand, L.O.; Tromberg, B.J.; Haskell, R.C.; Tsay, T.T.; Berns, M.W. Tissue characterization and imaging using photon density waves. Optical Engineering 1993, 32, 258–266. [Google Scholar] [CrossRef]
- Tromberg, B.J.; Svaasand, L.O.; Tsay, T.T.; Haskell, R.C. Properties of photon density waves in multiple-scattering media. Applied optics 1993, 32, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Fishkin, J.B.; Gratton, E.; VandeVen, M.J.; Mantulin, W.W. Diffusion of intensity modulated near-infrared light in turbid media. SPIE 1991, 1431, 122–135. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Boas, D.A.; Chance, B.; Yodh, A.G. Experimental images of heterogeneous turbid media by frequency-domain diffusing-photon tomography. Optics letters 1995, 20, 426–428. [Google Scholar] [CrossRef]
- Saikia, M.J. An embedded system based digital onboard hardware calibration for low-cost functional diffuse optical tomography system. SPIE 2021, 11632, 1–8. [Google Scholar] [CrossRef]
- Saikia, M.J.; Kanhirodan, R.; Vasu, R.M. High-speed GPU-based fully three-dimensional diffuse optical tomographic system. International Journal of Biomedical Imaging 2014, 2014. [Google Scholar] [CrossRef]
- Saikia, M.J.; Kanhirodan, R. High performance single and multi-GPU acceleration for Diffuse Optical Tomography. Institute of Electrical and Electronics Engineers Inc., 2014; pp. 1320–1323. [Google Scholar] [CrossRef]
- Saikia, M.J.; Rajan, K.; Vasu, R.M. 3-D GPU based real time Diffuse Optical Tomographic system. IEEE Computer Society, 2014; pp. 1099–1103. [Google Scholar] [CrossRef]
- Saikia, M.J.; Kanhirodan, R. Development of DOT system for ROI scanning. Optical Society of America (OSA), 2014, p. T3A.4. [CrossRef]
- Saikia, M.J.; Kanhirodan, R. Region-of-interest diffuse optical tomography system. Review of Scientific Instruments 2016, 87, 013701. [Google Scholar] [CrossRef]
- Saikia, M.J.; Besio, W.G.; Mankodiya, K. The Validation of a Portable Functional NIRS System for Assessing Mental Workload. Sensors 2021, 21, 3810. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, M.; Cay, G.; Saikia, M.J.; Mankodiya, K. Designing and testing a wearable, wireless fNIRS patch. Institute of Electrical and Electronics Engineers Inc., 2016; Volume 2016-Octob, pp. 6298–6301. [Google Scholar] [CrossRef]
- Saikia, M.J.; Mankodiya, K. A Wireless fNIRS Patch with Short-Channel Regression to Improve Detection of Hemodynamic Response of Brain. Institute of Electrical and Electronics Engineers Inc., 2018; pp. 90–96. [Google Scholar] [CrossRef]
- Saikia, M.; Mankodiya, K. 3D-printed human-centered design of fNIRS optode for the portable neuroimaging. 2019, 10870. [Google Scholar] [CrossRef]
- Saikia, M.; Besio, W.; Mankodiya, K. WearLight: Toward a Wearable, Configurable Functional NIR Spectroscopy System for Noninvasive Neuroimaging. IEEE Transactions on Biomedical Circuits and Systems 2019, 13. [Google Scholar] [CrossRef]
- Saikia, M.J.; Cay, G.; Gyllinsky, J.V.; Mankodiya, K. A Configurable Wireless Optical Brain Monitor Based on Internet-of-Things Services. Institute of Electrical and Electronics Engineers Inc., 2018; pp. 42–48. [Google Scholar] [CrossRef]
- Saikia, M.J. Internet of things-based functional near-infrared spectroscopy headband for mental workload assessment. SPIE 2021, 11629, 143–150. [Google Scholar] [CrossRef]
- Saikia, M.J.; Kuanar, S.; Borthakur, D.; Vinti, M.; Tendhar, T. A machine learning approach to classify working memory load from optical neuroimaging data. 2021, p. 69. [CrossRef]
- Saikia, M.J.; Brunyé, T.T. K-means clustering for unsupervised participant grouping from fNIRS brain signal in working memory task. SPIE, 2021, Vol. 11629, pp. 159–164. [CrossRef]
- Saikia, M.; Manjappa, R.; Kanhirodan, R. A cost-effective LED and photodetector based fast direct 3D diffuse optical imaging system. 2017, Vol. 10412. [CrossRef]
- Saikia, M.J.; Mankodiya, K.; Kanhirodan, R. A point-of-care handheld region-of-interest (ROI) 3D functional diffuse optical tomography (fDOT) system. SPIE, 2019, Vol. 10874, p. 90. [CrossRef]
- Saikia, M.J.; Kanhirodan, R. A tabletop Diffuse Optical Tomographic (DOT) experimental demonstration system. SPIE, 2019, Vol. 10869, p. 11. [CrossRef]
- Saikia, M.J.; Manjappa, R.; Mankodiya, K.; Kanhirodan, R. Depth sensitivity improvement of region-of-interest diffuse optical tomography from superficial signal regression. OSA - The Optical Society, 2018, Vol. Part F99-C, p. CM3E.5. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



