Submitted:
12 January 2023
Posted:
17 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
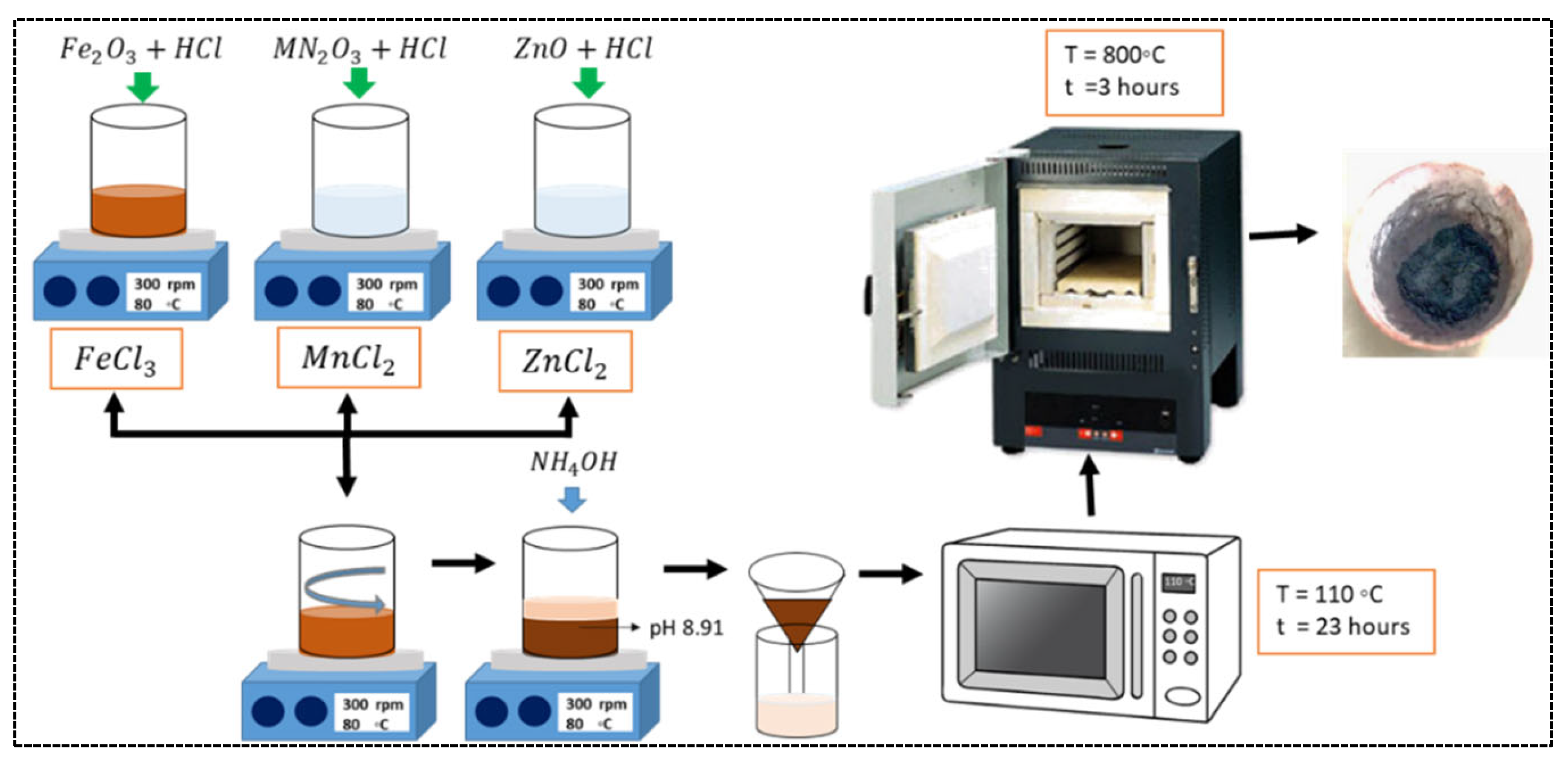
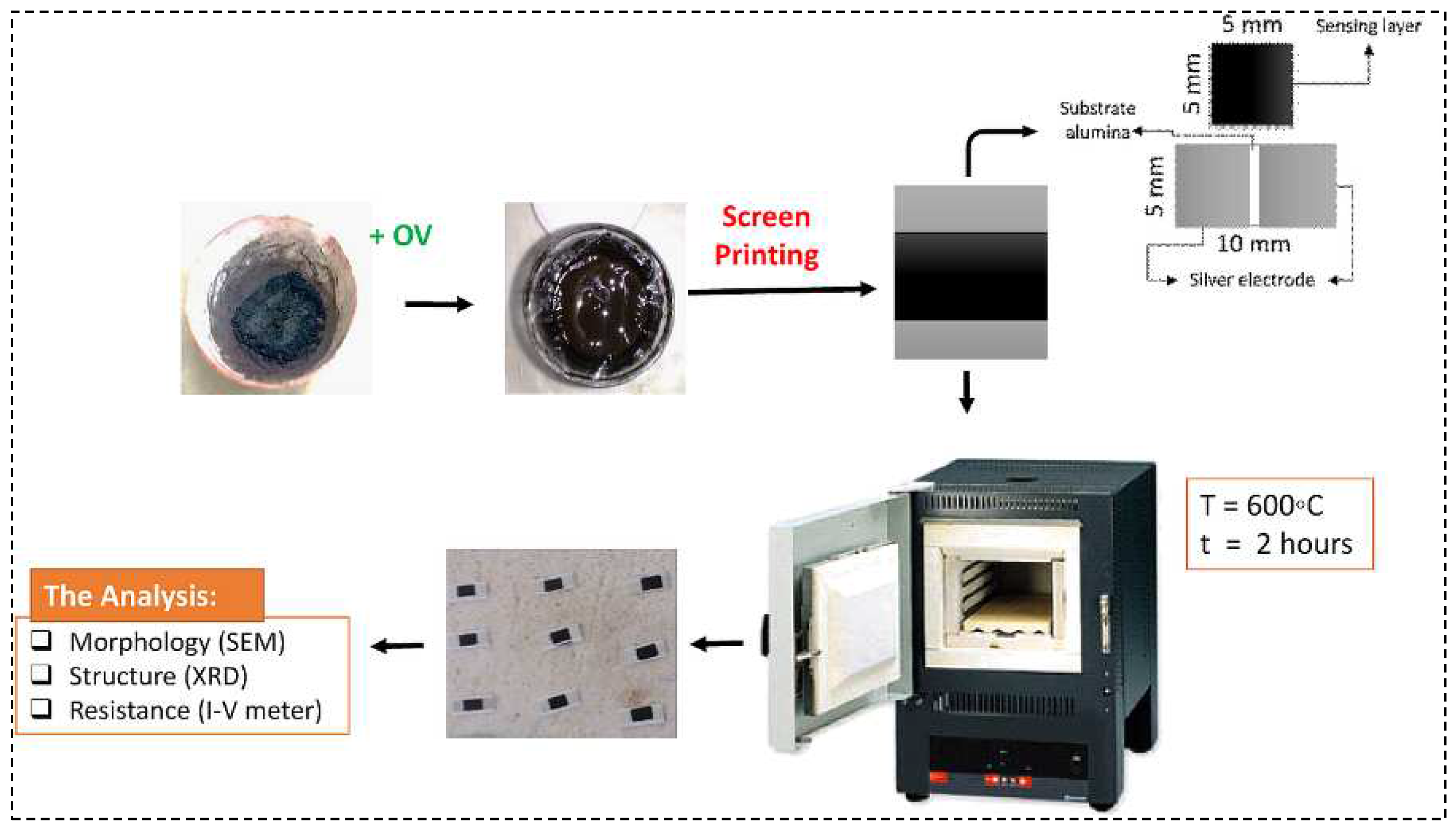
2. Materials and Methods
Preparations of Composite Powdersby Precipitation
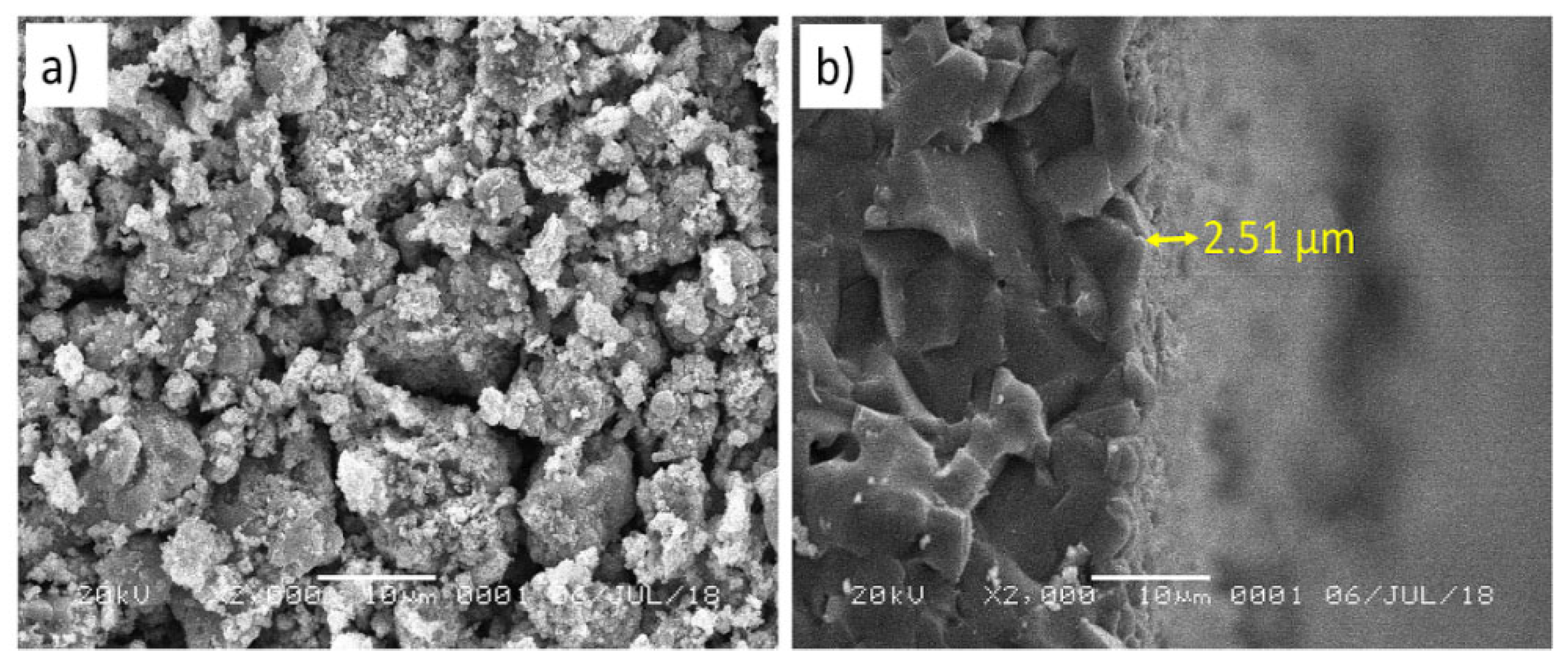
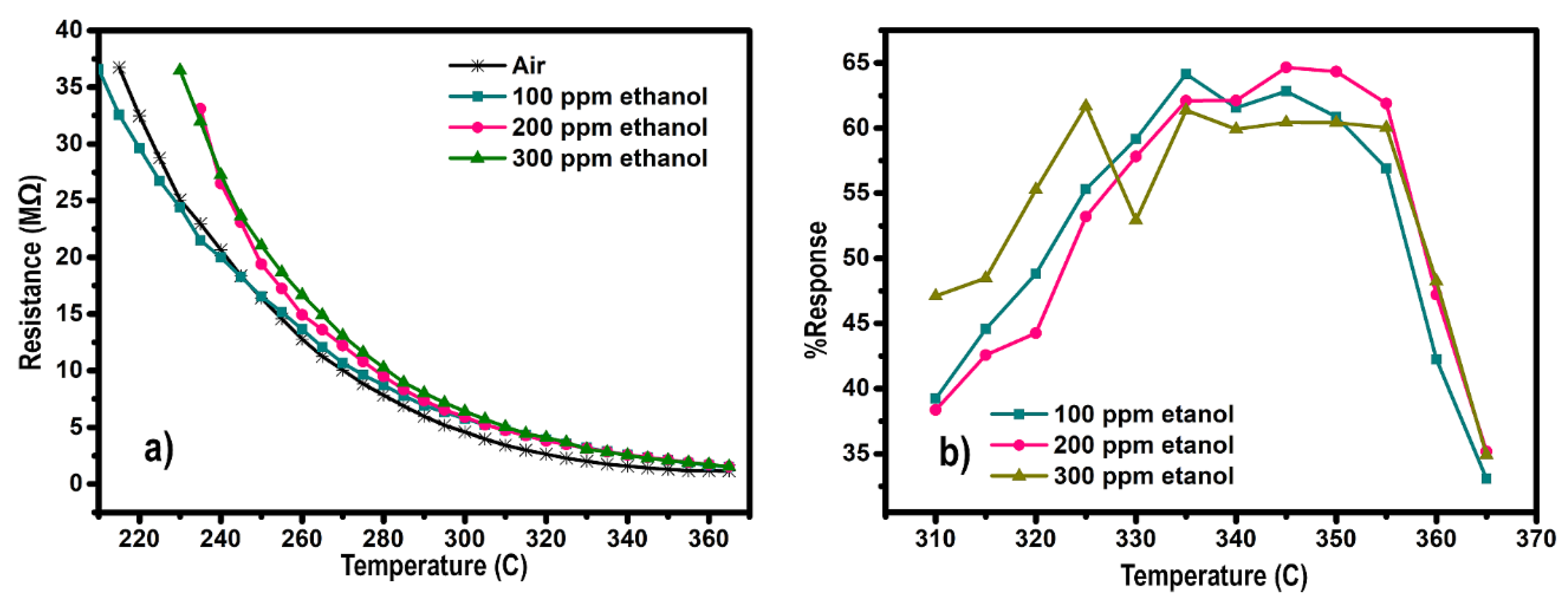
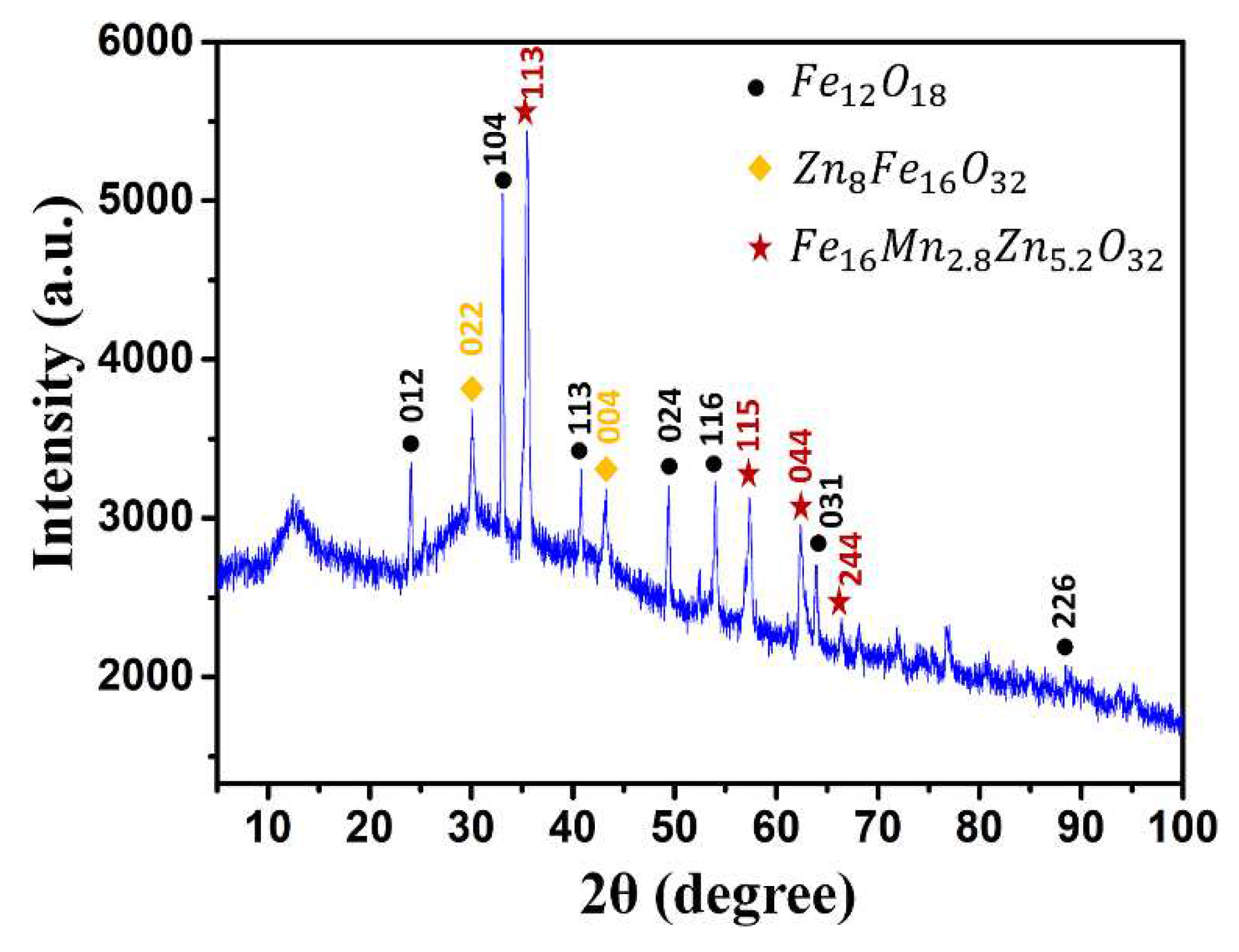
3. Results and Discussion
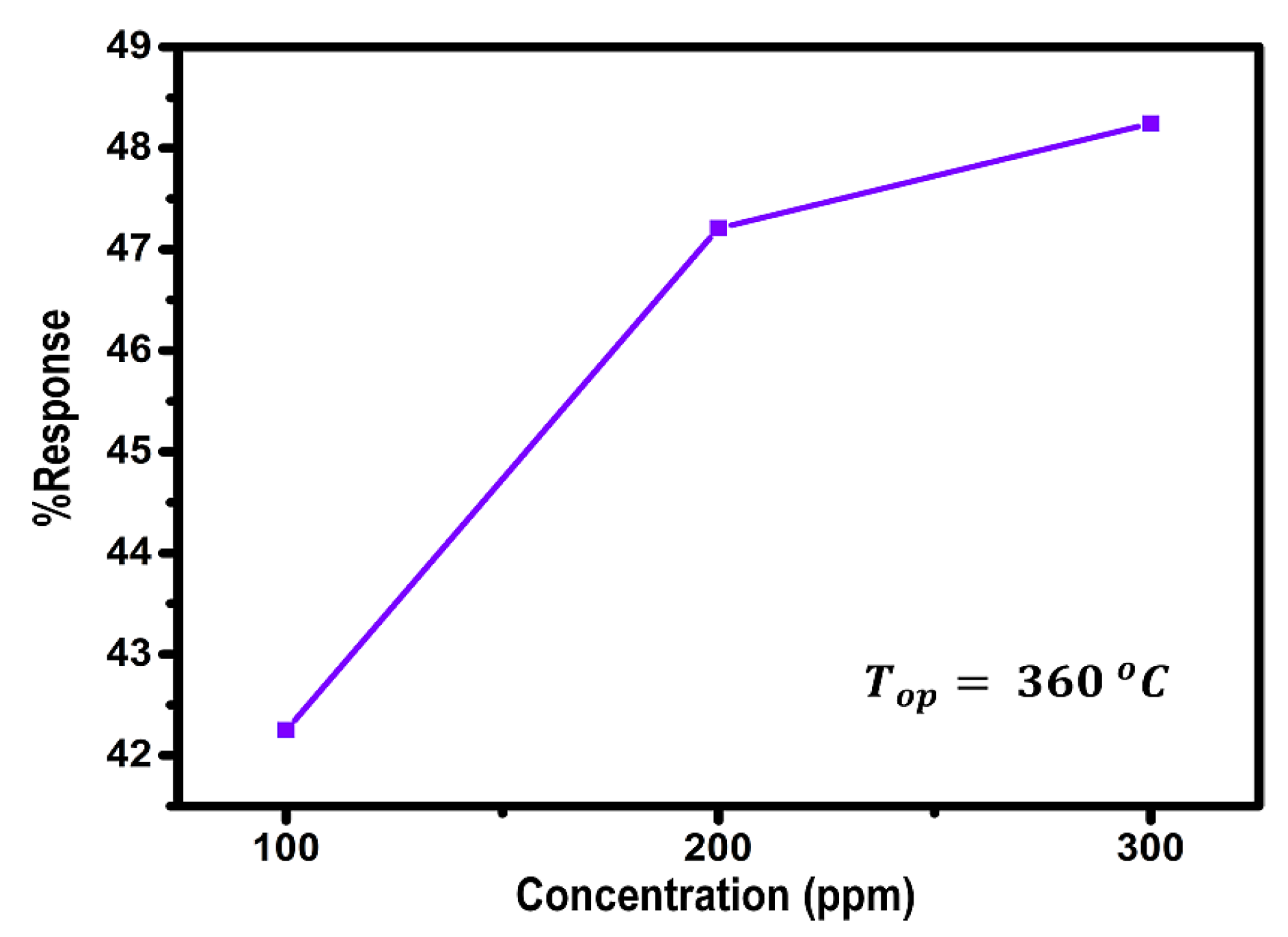
3.1. Sensing Behavior as an Indicator for Gas Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- S. Sharma, P. Chauhan, and S. Husain, “Liquefied petroleum gas sensor based on manganese (III) oxide and zinc manganese (III) oxide nanoparticles,” Mater. Res. Express, vol. 5, p. 015014, 2017. [CrossRef]
- X. F. Wang et al., “Prussian Blue analogue derived porous NiFe2O4 nanocubes for low-concentration acetone sensing at low working temperature,” Chem. Eng. J., vol. 338, no. October 2017, pp. 504–512, 2018. [CrossRef]
- S. Ameen, “Vertically arranged Mn2O3 nanosheets as smart sensing electrode for highly sensitive N-hydroxysuccinimide,” Microchem. J., vol. 163, p. 105912, 2021. [CrossRef]
- D. Michele et al., “Opto-Electronic Characterization of Photocatalysts Based on p,n Junction Ternary and Quaternary Mixed Oxides Semiconductors (Cu2O-In2O3 and Cu2O-In2O3-TiO2),” Catalysts, vol. 12, pp. 1–22, 2022.
- N. F. Samsudin, K. A. Matori, J. Y. C. Liew, Y. W. Fen, M. H. Mohd Zaid, and Z. N. Alassan, “Investigation on structural and optical properties of willemite doped Mn2+ based glass-ceramics prepared by conventional solid-state method,” J. Spectrosc., vol. 2015, 2015. [CrossRef]
- D. G. Syarif, D. H. Prajitno, and E. Umar, “Synthesis of Al2O3 nanoparticles from Local Bauxite for water-Al2O3 nanofluids egy,” in Journal of Physics: Conference Series, 2017, vol. 799, p. 012014.
- H. Wang and P. P. Mercier, “Near-Zero-Power Temperature Sensing via Tunneling Currents Through Complementary Metal-Oxide-Semiconductor Transistors,” Sci. Rep., vol. 7, no. 1, pp. 1–7, 2017. [CrossRef]
- Y. Shan, M. Y. Zhang, Y. Bai, M. Du, X. Guo, and H. Pang, “Design and synthesis of transition metal oxide/zeolitic imidazolate framework-67 composites,” Chem. Eng. J., vol. 429, no. June 2021, p. 132146, 2022. [CrossRef]
- D. Kaya, H. S. Aydınoğlu, E. Şenadım Tüzemen, and A. Ekicibil, “Investigation of optical, electronic, and magnetic properties of p-type NiO thin film on different substrates,” Thin Solid Films, vol. 732, no. February, p. 138800, 2021. [CrossRef]
- H. Aliah et al., “Structure analysis of nanocomposite ZnO:Fe2O3 based mineral yarosite as Fe2O3 source and its application probability,” in Materials Today: Proceedings, 2019, vol. 13, pp. 36–40.
- H. Aliah et al., “Semiconductor Ceramic Mn0.5Fe1.5O3-Fe2O3 from natural minerals as ethanol gas sensors,” in IOP Conference Series: Materials Science and Engineering, 2018, vol. 367, no. 1, pp. 0–6.
- D. T. Dimitrov, “From gas sensors to detection of etanol vapour to sensor of bacteria detection,” J. Russ. Univ. Radioelectron., vol. 22, no. 5, pp. 93–106, 2019. [CrossRef]
- H. Aliah, D. G. Syarif, R. N. Iman, W. Darmalaksana, A. Setiawan, and A. Sawitri, “Preliminary studies of Mn(x)Zn(2-x)FeO4 (x = 0 dan 0.1) thick film semiconductors as an ethanol gas sensor,” in IOP Conference Series: Materials Science and Engineering, 2018, vol. 434, no. 1, pp. 0–7.
- [C. M. Hung, N. D. Hoa, N. Van Duy, N. Van Toan, D. T. T. Le, and N. Van Hieu, “Synthesis and gas-sensing characteristics of α-Fe2O3 hollow balls,” J. Sci. Adv. Mater. Devices, vol. 1, no. 1, pp. 45–50, 2016.
- K. Fan, J. Guo, L. Cha, Q. Chen, and J. Ma, “Atomic layer deposition of ZnO onto Fe2O3 nanoplates for enhanced H2S sensing,” J. Alloys Compd., vol. 698, pp. 336–340, 2017. [CrossRef]
- N. T. A. Thu et al., “Fe2O3 nanoporous network fabricated from Fe3O4/reduced graphene oxide for high-performance ethanol gas sensor,” Sensors Actuators, B Chem., vol. 255, pp. 3275–3283, 2018.
- Y. Chen et al., “ZIF-8 derived hexagonal-like α-Fe2O3/ZnO/Au nanoplates with tunable surface heterostructures for superior ethanol gas-sensing performance,” Appl. Surf. Sci., vol. 439, pp. 649–659, 2018.
- B. Zhang, W. Fu, X. Meng, A. Ruan, P. Su, and H. Yang, “Synthesis and enhanced gas sensing properties of flower-like ZnO/α-Fe2O3 core-shell nanorods,” Ceram. Int., vol. 43, no. 8, pp. 5934–5940, 2017.
- Y. LI, L. li CHEN, and F. xian ZHAO, “Highly selective acetone sensor based on ternary Au/Fe2O3-ZnO synthesized via co-precipitation and microwave irradiation,” Trans. Nonferrous Met. Soc. China (English Ed., vol. 28, no. 1, pp. 137–144, 2018. [CrossRef]
- J. Ma et al., “High sensitivity and ultra-low detection limit of chlorine gas sensor based on In2O3 nanosheets by a simple template method,” Sensors Actuators, B Chem., vol. 305, no. September, p. 127456, 2020.
- E. Suhendi, A. E. Putri, M. T. Ulhakim, A. Setiawan, and D. G. Syarif, “Investigation of ZnO doping on LaFeO3/Fe2O3 prepared from yarosite mineral extraction for ethanol gas sensor applications,” AIMS Mater. Sci., vol. 9, no. 1, pp. 105–118, 2022.
- S. Bai et al., “Reverse microemulsion in situ crystallizing growth of ZnO nanorods and application for NO2 sensor,” Sensors Actuators, B Chem., vol. 190, pp. 760–767, 2014. [CrossRef]
- S. D. Shinde, V. B. Gaikwad, G. E. Patil, D. D. Kajale, and G. H. Jain, “Gas sensing performance of nanostructured ZnO thick film resistors,” Int. J. Nanoparticles, vol. 5, no. 2, pp. 126–135, 2012. [CrossRef]
- Y. Ma, Y. Lu, H. Gou, W. Zhang, S. Yan, and X. Xu, “Octahedral NiFe2O4 for high-performance gas sensor with low working temperature,” Ceram. Int., vol. 44, no. 2, pp. 2620–2625, 2018. [CrossRef]
- V. B. Kamble and A. M. Umarji, “Gas sensing response analysis of p-type porous chromium oxide thin films,” J. Mater. Chem. C, vol. 1, no. 48, pp. 8167–8176, 2013. [CrossRef]
- M. Gardon and J. M. Guilemany, “A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors,” J. Mater. Sci. Mater. Electron., vol. 24, no. 5, pp. 1410–1421, 2013. [CrossRef]
- B. Xiao et al., “Mixed potential type ammonia sensor using Fe-substituted LaCoO3 sensing electrode prepared by flame spray pyrolysis,” Sensors Actuators, B Chem., vol. 346, no. June, p. 130470, 2021. [CrossRef]
- A. K. Srinath, L. Sankaranarayanan, R. Pandeeswari, and B. G. Jeyaprakash, “Thin films of α-Mn2O3 for resistance-based sensing of acetaldehyde vapor at ambient temperature,” Microchim Acta, vol. 182, pp. 1619–1626, 2015. [CrossRef]
- N. N. Jandow, A. K. Elttayef, A. F. Majied, N. F. Habubi, N. Saadeddin, and Y. Al-Douri, “Thickness effect of ZnO/PPC gas sensor on the sensing properties of NO2 gas,” AIP Conf. Proc., vol. 2083, no. 2, 2019.
- N. Yamazoe, “Toward innovations of gas sensor technology,” Sensors Actuators, B Chem., vol. 108, no. 1-2 SPEC. ISS., pp. 2–14, 2005. [CrossRef]
- L. Yuan, T. Hyodo, Y. Shimizu, and M. Egashira, “Preparation of mesoporous and/or macroporous SnO2-based powders and their gas-sensing properties as thick film sensors,” Sensors, vol. 11, no. 2, pp. 1261–1276, 2011. [CrossRef]
- H. Aliah et al., “The optimization of ZnFe2O4/Mn2O3-based nanocomposite ceramic fabrication utilizing local minerals as an ethanol gas detector,” Mater. Res. Express, vol. 6, no. 9, p. 95908, 2019.
- A. C. Doriguetto and N. G. Fernandes, “Manganese‐rich natural franklinite,” Acta Crystallogr., vol. C55, pp. 1751–1753, 1999. [CrossRef]
- D. Levy, A. Pavese, and M. Hanfland, “Phase transition of synthetic zinc ferrite spinel (ZnFe2O4) at high pressure, from synchrotron X-ray powder diffraction,” Phys. Chem. Miner., vol. 27, no. 9, pp. 638–644, 2000.
- R. L. Blake and R. E. Hessevick, “Refinement of the hematite structure,” Am. Mineral., vol. 51, pp. 123–129, 1966.
- P. Puspitasari, N. Yahya, N. A. M. Zabidi, and N. A. Ahmad, “Comparison of Mechanical Properties and Magnetic Properties of Mn0.8Zn0.2Fe2O4 Synthesized by Conventional Ball Milling and Self Combustion Method,” J. Appl. Sci., vol. 1999, no. December, pp. 1–6, 2006. [CrossRef]
- S. Zhu, H. Sun, X. Liu, J. Zhuang, and L. Zhao, “Room-Temperature NH3 sensing of graphene oxide film and its enhanced response on the laser-Textured silicon,” Sci. Rep., vol. 7, no. 1, pp. 1–8, 2017. [CrossRef]
- H. Qin et al., “Improved thermoelectric performance of p-type Bi0.5Sb1.5Te3 through Mn doping at elevated temperature,” Mater. Today Phys., vol. 6, pp. 31–37, 2018.
- Y. Jamil, M. R. Ahmad, A. Hafeez, Z. Ul Haq, and N. Amin, “Microwave assisted synthesis of fine magnetic manganese ferrite particles using co-precipitation technique,” Pak. J. Agri. Sci, vol. 45, no. 3, pp. 59–64, 2008.
- G. Zhang and M. Liu, “Effect of particle size and dopant on properties of SnO2-based gas sensors,” Sensors Actuators, B Chem., vol. 69, no. 1, pp. 144–152, 2000. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




