1. Introduction
Childhood malnutrition is one of the primary persistent community health challengesall through developing countries as well as in Nigeria. Geographic and wellness survey data from twenty-one developing countries suggested that impoverished complementary feeding of infants aged 6-23 months adds to unfavourable growth trends [
1]. In sub-Saharan African countries, poor infant eating habit as well as impoverished nutritional attributes of complementary foods, micronutrient deficiencies coexisting withpersistent infections adds to high mortality rates among infants and young children [
2]. Malnutrition for example, has been accountable for directly or indirectly 10.9 million (60%) of the global yearly under five infant deaths [
3]. Therefore to decrease the issue of malnutrition amid children in the public, development of complementary food ample in essential nutrient for maximum growth and development of infants, is crucial. Complementary food has been defined as any healthful and energy containing solid, semisolid or liquid meal consumed by infants besides human milk or formula [
4]. Complementary foods are essentially introduced from the period of 4-6 months when breast milk considered being the choicest and safest meal for young babies, can no longer provide the nutrients and energy requirements needed to enable the child to grow and thrive.Given the comparative insignificant amounts of complementary foods that are ingested at 6-24 months, the nutrient bulkiness of complementary foods needs to be very high to satisfy these needs. One of the most important ways of improving nutritional status of a food is primarily through fortification. This could be achieved by developing foods made from different blends of plant produce to enhance the nutritional benefits of the final products. Therefore, this work is aimed at investigating the use of solid-state fermentation to enhance the nutritional quality of
fonio (acha), soyabean and orange-fleshed sweet potatoes utilizing
Rhizopusoligosporus and Lactobacillus planterum thereby enhancing their suitability for use as complementary foods.
2. Materials and Method
Procurement of Raw Materials
Fonio/acha (Digitariaexilis) and soybeanwas obtained from Ogige market, Nsukka in Enugu state, Nigeria, Orange fleshed sweet potatoes was procured from a research institute in Yenogoa, Bayelsa state, Nigeria, bacterial culture: Rhizopusoligosporus(2710) and Lactobacillus plantarum(B-41621) was obtained from the United States Department of Agriculture (USDA), Agricultural Research Service, ARS culture collection centre, USA.
Culture Preparation
The organisms, Lactobacilliusplantarum (B-41621)and Rhizopusoligosporus (2710) obtained from USDA were preserved in a dormant state inside tubes. A file scratch was made in the centre of the tube and wiped with 70% alcohol. The tube was broken open using a paper towel to cushion hands. The pellet was transferred to approximately 1-2ml suitable broth medium and was allowed to dissolve for several minutes. It was finally homogenized by finger-vortex before using the suspension as inoculum.
Preparation of Raw Materials
One kilogram of the unprocessed materials were immersedsingly each in four (4) volumes of 0.9M acetic acid for 16 h, cleanse with clean water, steam cooked for 7 minutes at 121°C, allowed to cool at room temperature. Solid state fermentation (SSF) was achieved by inoculating Fonio and Soybean with spore suspension (1×106spores/ml) of Rhizopusoligosporus (2710) and OFSP with spore suspension (1×106spores/ml) of Lactobacillus planterum(B-41621), allowed to ferment for 72hours, after which it was dried at 50°C for 24hours, milled and sieved. The samples were blended in the following ratios: Fonio and Soybean 100: 100 (AS), fonio/soybean and OFSP 50: 50(ASO), and made into gruel.
Proximate Composition
Moisture content, crude fibre, ash, protein, fat were determined using [
5] method while carbohydrate content was determined by difference.
Determination of Selected Micronutrient Content of the Samples
Determination of Iron using Atomic Absorption Spectrophotometer (AAS).
Sample Preparation
Iron was determined using AAS as described by [
5]. One gram (1g) of the sample was first digested with 30ml of aqua regia which is a mixture of concentrated HNO
3 and HCL in the ratio of 1: 3. The digested sample was filtered and made up to 50ml with deionized water. The aliquots of the digested filtrate were used for AAS using filters that match the different elements.
Determination of Beta-Carotene
Beta-carotene was determined using the method [
5]. One gram (1 g) of the sample each was extracted by mixing with 20 ml of petroleum ether. The extract was evaporated to dryness and the residue dissolved with 0.2 ml chloroform-acetic anhydride mixture. 2 ml of trichloro-acetic acid (TCA) was also added to the extract mixed thoroughly and the absorbance read at 620 nm within 15 seconds. With the absorbance value, beta-carotene was calculated thus:
Abs = Absorbance
Df = Dilution factor
E = Extinction coefficient
Determination of Vitamin C
Vitamin C content was determined according to the method of [
6]. Five gram of the sample was weighed into a 100ml volumetric flask, 2ml of 20% meta-phosphoric acid was added as stabilizing agent and the solution was diluted to volume with distilled water. Ten (10) ml of the solution was pippeted into a small flask and 2.5ml of acetone added. The solution was titrated with indophenols solution until a faint pink colour persisted for 15 seconds. The vitamin C content was calculated as mg/100ml.
Determination of Zinc
Zinc content of the sample was determined according to [
5]. Five (5) ml of the filtrate was pipette into duplicate tubes to which 4.6 ml of actetateacetic acid buffer solution was added followed by gentle shaking 10 minutes. Dithizone (0.4 ml) was added and the pH adjusted to 4.5 with 20 % N
aOH before the absorbance was taking at 520 nm in a spectrophotometer.
Determination of Calcium
The calcium content was determined according to the method of [
5]. One millilitre of the filtrate was pipette into duplicate tubes, then 3 ml calcium working reagent consisting of dye solution salt (0.18 g) methythymol blue, 6.0 polyvinyl pyrolidone, 7.2 g hydroxyquinoline, 10 ml hydrochloric acid concentrated, 1 litre of distilled water) was added and shaken for 10 minutes, absorbance was taken at 612 nm against a blank using a spectrophotometer.
Determination of Vitamin B1 (Niacin)
Thiamine was determined by using [
5]. Five grams (5 g) of the samples were homogenized in 5ml normal ethanoic sodium hydroxide solution. The homogenate was filtered and made up to 100ml with the extract solution. Ten millilitres (10 ml) aliquot of the extract was dispensed into a flask and 10ml of potassium dichromate solution were added. The resultant solution was incubated for 15minutes at room temperature (25±1℃). The absorption was obtained from the spectrophotometer at 360 nm using a reagent blank to standardize the instrument at zero. The thiamine content was calculated as follows
Thiamine mg/100g= 100 x au x C x d
was
Microbial Analysis
Total Viable Count
Pour plate method as described by [
7] was used. One gram of the sample was macerated into 9ml of Ringers solution and mixed thoroughly by shaking. Then 0.1ml dilution was transferred from each dilution bottle into the corresponding plate and 15ml of sterile nutrient agar medium was poured and mixed thoroughly with the inoculum by rocking the plates. The plates were incubated at 38
0C for 24hours after which the colonies formed were counted and expressed as colony forming units per gram (cfu/g).
Mould Count
The pour plate method as described by [
7] was also used. The sample dilution weighing 0.1ml was transferred from each dilution into corresponding plates and 15ml of sterile Sabourand Dextrose Agar (SDA) medium was poured and mixed thoroughly with the inoculum by rocking the plates. The plates were incubated at ambient temperature for three days after which colonies formed were counted and expressed as colony forming units per gram (cfu/ml).
Sensory Evaluation
The complementary food samples were evaluated for colour, taste, texture, mouth feel, aftertaste and overall acceptability on a 9-point Hedonic Scale, where 1=dislike extremely, 5= neither like nor dislike, and 9= like extremely as described by [
8]. The evaluation was done by a 20- man panellists selected randomly from among nursing mothers and pregnant women of Ziks Flat University of Nigeria, Nsukka.
Statistical Analysis
Data analysis was done using one-way analysis of variance (ANOVA) based on completely randomized design (CRD). Mean separation was done using Duncan New Multiple Range Test using SPSS version 23.0 computer software.
3. Results AND Discussion
Effect of Fermentation (SSF) Time on the Proximate Composition (%) of the Fermented Raw Material
Table 1 shows the effect of fermentation (SSF) time on the proximate composition of the fermented raw materials.
The moisture content of the fermenting raw materials ranged from 54.97 – 56.27% with sample AS
0 having the least moisture content and AS
72 the highest value. From the result, it can be deduced that as fermentation progressed, there was an increase in moisture content. The increase in moisture content is consistent with the findings of [
9] who observed that fermentation increased the moisture content of pigeon pea flour. This could be credited to the catabolic breakdown of substrates due to fermentation which releases water. There were significant (P<0.05) differences among the samples.
The protein content of the fermenting raw materials ranged from 17. 10 – 19.02% with sample AS
0 having the least protein value and sample AS
72 having the highest protein content. There was a gradual increase in the protein content as the fermentation time increased. There was a significant (p<0.05) difference among the protein content of the samples at different fermentation time. The observed increase in protein content of the samples is similar to that observed by [
10]. He reported that solid state fermentation (SSF) increased the protein content of common bean flour to 21.7%.
The fat content of the fermenting raw materials ranged from 5.81– 4.52% with sample AS
0 having the highest value and AS
72 having the least value. The fat content of the fermented samples decreased with increasing fermentation time (
Table 1). Ruiz-Teran and Owen [
11],reported that during SSF of soybean a considerable depletion in crude lipids takes place during the initial stages of fermentation. They attributed this reduction to the oxidation and utilisation of fatty acids by the fungus as a source of energy. Fatty acids present in glycerides have been reported to decrease during fermentation of soy bean from 30% natural lipid by the action of lipases activity [
12]. [
13] reported a reduction in the fat content of Barley during SSF by
R.oligosporus from 2.13- 1.62%.
The ash content of the blends ranged from 2.09 – 2.38% with sample AS
0 having the least ash content value and AS
72 having the highest value. The ash content is the index of the mineral content of food samples which is vital for infant growth and development. There was significant (p<0.05) difference between the samples. The ash content increased as the fermentation time increased. This observation is in agreement with that of [
14], who observed an increase in ash content of fermented maize-cowpea blends.
The carbohydrate content of the fermenting raw materials ranged from 12.95 – 10.21% with AS
0 having the highest value and AS
72 having the least value. The carbohydrate content of the samples decreased with increased fermentation time. A decrease in carbohydrate level during fermentation could be due to the incomplete removal of non-starch component in the course of solid state fermentation process. It has also been reported that during the fermentation process of cereals, proteases, lipases, phytases and a variety of carbohydrases are created resulting in the breaking down of macromolecules into lower weight products thereby enhancing the nutritional quality of fermented product [
12]. There was significant (p>0.05) difference among the samples.
The pH of the fermenting samples decreased as the fermentation progressed from 0 hour to 72 hours of fermentation. This decrease in pH is attributed to the production of organic acids in the fermenting samples. A similar result was observed by [
15]. The pH ranged from 4.72 – 3.11 with sample AS
0 having the highest value and AS
72 having the least value. There were significant (p<0.05) differences among the samples.
Titratable acidity (TTA) increased with time over the entire fermentation period. A similar increase in acid production was observed by [
14] during the production of weaning food from maize-cowpea blends. The increase in acidity is of great significance as it is reported to reduce the incidence of diarrhoea in infants.
Effects of SSF on the Functional Properties of the Complementary Food
The functional properties of complementary food formulated from blends of Fonio, soybean and orange-flesh sweet potato flour are shown in
Table 3.
The viscosity ranged from 8200±1.71 - 15400±0.71cP with sample CTRL (commercial product) having the least value and sample ASO having the highest value. There were significant (p<0.05) differences among the samples. Their variations in viscosity could be due to the effect of Solid-State Fermentation as SSF could be applied in the modification of flour. Lactic acid bacteria fermentation enhanced the viscosity of Kefir grain flour. The kefiran produced increased the binding ability of kefir grain flour with water and increased interaction of flour with water in the presence of protein [
24].
Water absorption capacity represents the ability of a product to associate with water under conditions where water is limited [
25]. It is desirable for food systems to improve yield and consistency and to give body to the food. The values for water absorption capacity ranged from 450 – 551.3%. There were significant (p<0.05) difference between sample CTRL (commercial product) and the other samples. Samples AS and ASO had the highest content as a result of the effect of SSF on the flour blends. SSF increased the water absorption index of QPM flour from 1.25 to 2.93g gel/g dry flour [
16]. Water absorption capacity is a critical function of protein in various food products like soups, dough and baked products [
26]. Increase in protein content, had a positive influence on the water absorption capacity of the samples.
The swelling capacities of the samples ranged from 2.25 – 3.31% with sample AS having the highest value and sample CTRL(commercial product) having the least value. High swelling capacity value of sample AS and ASO could be due to the effect of SSF on the sample. There were significant (p<0.05) difference among the samples. SSF increased the swelling power and solubility of finger millet flour from 12 to 20.04 and from 17.25 to 13.25 due to change in gelatinization properties of the flour [
27].
Micronutrient Content of the Formulated Complementary Food
Minerals
Table 4 shows the mineral and vitamin composition of the complementary food formulated from blends of Fonio, soybean and orange-flesh sweet potato flour.
The iron content of the complementary food ranged from 6.57 – 8.41mg/100g with sample ASO having the least value and sample CTRL (commercial product) having the highest value. Although sample CTRL had the highest value due to fortification, samples AS and ASO also contained good amounts as Soybean which is a constituent of the formulated food is a rich plant source of Iron. Iron is crucial for cognitive development and transportation of oxygen in the body [
28]. The recommended daily allowance for iron intake by infants is between 0.27 and 11mg [
29]. All the samples contained acceptable quantities of iron when compared to the recommended daily allowance. There was a significant (p>0.05) difference among the samples.
Calcium is necessary for optimal growth and development of infants and young children [
30]. The calcium content of the complementary food ranged from 96.23-327.12mg/100g with sample ASO having the least value and sample CTRL (commercial product) having the highest value. The samples contained significant amount of the element and as such makes it an ideal meal for children and adults alike.
Zinc is also beneficial to children with diarrhoea because is an indispensable micronutrient necessary for protein synthesis, cell growth, and differentiation, immune function and intestinal transport of water and electrolytes [
31]. The Zinc content in this work ranged from 2.43-5.52mg/100g with samples CTRL (commercial product) having the highest value and sample ASO having the least value. The recommended daily intake for zinc in infants (6 – 12 months) is 0.6mg [
32], and this values observed in this study is higher than the recommended zinc intake.
The vitamin A content of the complementary food blend ranged from 1134-2560(µg/100g) with sample ASO having the highest value and sample AS the least value. There was significant (p<0.05) difference among the samples. Sample ASO had the highest content because it contained orange-flesh sweet potato which is a rich source of Pro Vit A. One of the easiest ways to introduce more Vitamin A into an infant’s diet is by addition of carotene-rich plant based foods. Vitamin A is an essential nutrient that helps build up the immune system of infants against a number of infections and sustains the integrity of the epithelial linings [
33]. These values were higher than 1380.00 to 1623.33(µg/100g) reported by [
34]) for moringa-fortified orange fleshed sweet potato complementary food.
The Vitamin C content ranged from 18.32 – 65.07mg/g with sample CTRL (commercial product) having the highest value and sample AS having the least value. There were significant (p<0.05) difference among the samples and their content were generally low. Vitamin C helps to form and repair red blood cells, bones, and tissues; it also helps cut and wounds to heal, boosts the immune system and keep infections away. The RDI of Vitamin C for infants 7-12 months is 50mg/ day. Sample AS and ASO were less than the required intake but sample CTRL (commercial product) was above the RDI, this could be because it is a fortified food.
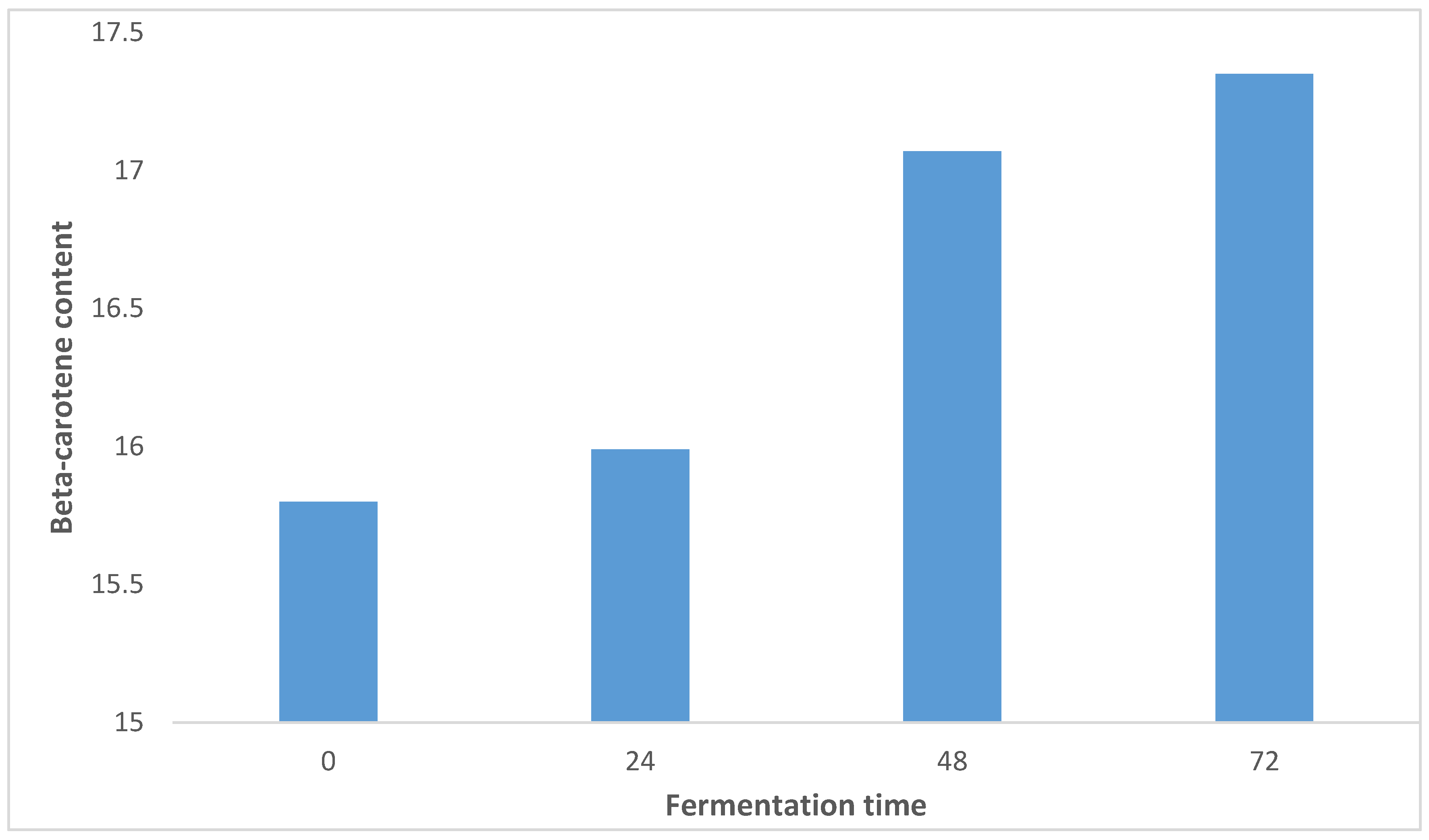
Effect of SSF on the Retention of Beta-Carotene Content in OFSP
Sweet potatoes, especially orange-fleshed sweet potatoes (OFSP) varieties contain significant amounts of β-carotene (CIP, 2017). The effect of SSF on the retention of β-carotene content in OFSP is shown in
Figure 1. The content ranged from 15.80-17.35 (µg β-carotene/g) with sample OFSP0 having the least value and sample OFSP72 with the highest value. There was significant (p˃0.05) difference between the samples. The β- carotene content increased as fermentation time increased. This was similar to the finding of [
35], who reported that lactic acid fermentation using
Lactobacillus plantarum produced Lacto pickles from Zapallo OFSP with 93.97% β-carotene retention and adequate shelf-life.
Effects of Fermentation (SSF) Time on the Total Viable Count and Mold Counts of the Fermenting Raw Materials
Table 5 shows the effect of fermentation time on the total viable and mould count of the fermenting samples
Total viable count gives a quantitative idea about the presence of microorganisms in the sample The total viable count (cfu/ml)
) for the blends ranged from 2.8x10
5 (AS
0), 2.2x10
5 (AS
24), 4.0x10
4 (AS
48) to 1.7x10
4 (AS
72) while the values for the finished products ranged from 2.0x10
4 (AS) to 2.4x10
4 cfu/ml(ASO). It was observed that the value was highest on the 48th hour. This could be as a result of the microbial organisms growing and multiplying as that was the peak of the fermentation period. After 48 hours of fermentation period, the microbial count of the blends decreased gradually. In the final product, it was observed that the TVC count increased. This could be as a result of the mild heat treatment used in drying which still allows the microbial strains to thrive. The microbial strains are beneficial to the intestinal guts of children as they are probiotic. However, the samples were within safe limits recommended for foods [
36].
The Mold count in (cfu/ml)
) for the fermented complementary ranged from 2.0×10 (AS0), 4.0×10 (AS24), 2.0×10 (AS48) to 1.0×10 (AS72). Mold count was recorded throughout the fermentation time. This was as a result of the microbial strain (
Rhizopusoligosporus) used to ferment the samples which is a Fungus. The growth of mold during the fermentation process signifies that the fermentation is progressing. The mold count increased at the 24th hour of fermentation and decreased on the 72nd hour. On the final product, the mould count ranged from 1.8×10-2.0×10. This could be as a result of the mild heat treatment used in drying which still allows the microbial strains to thrive. The microbial strains are beneficial to the intestinal guts of children as they are probiotic. The microbial contents in the samples were within safe limits recommended for foods. [
36].
4. Conclusion
Complementary food produced by solid-state fermentation of Fonio, Soybean and Orange-fleshed sweet potatoes, improved the protein, fibre and ash contents while the carbohydrate content decreased. SSF improved the Pro-vitamin A content of the food by the addition of OFSP and also improved some of the mineral content of the formulated food such as Vitamin A and B1. Although the commercial sample (Cerelac Maize+Soya) was most preferred, the sensory scores indicated that sample AS, (100% fonio/soybean blend) is recommended compared to other blend as there was no significant (p<0.05) difference between the sample and the control. This study showed that acceptable physicochemical and nutritious weaning food can be produced from Solid-state fermentation of Fonio, Soybean and OFSP. The low moisture medium and use of beneficial microorganisms Rhizopusoligosporus(2710) and Lactobacillus plantarum(B-41621), improved the nutritional content of the formulated food, will act as probiotics in the intestinal guts of the children and may reduce childhood illnesses such as diarrhoea. OFSP can be a year round source of Vitamin A in developing countries.
Author Contributions
Supervision: Ngozi C. Okoronkwo; Writing-Review & Editing: Chigozie F. Okoyeuzu; Conceptualization: Ngozi C. Okoronkwo & Ifeoma E. Mbaeyi-Nwaoha; Investigation: Chidinma P. Agbata; Methodology& Software: Chinwe R. Eze.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgements
The authors, wish to acknowledge the assistance of the United States Department of Agriculture (USDA), Agricultural Research Service, ARS Culture Collection Centre, USA who provided the microbial culture used in this research work. Your impact cannot be overemphasized.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dewey, K. (2003). Guiding principles for complementary feeding of the breastfed child.
- Black, R. E, Allen, L. H., Bhutta, Z. A., Caulfield, L. E., De Onis, M. and Ezzati, M. (2008). Maternal and Child Under-nutrition: Global and Regional Exposures and Health Consequences. The Lancet, 371(9608): 243-260. [CrossRef]
- WHO and UNICEF. (2003). Global Strategy for Infant and Young Child Feeding: World Health Organisation. https://www.who.int/publications/i/item/9241562218.
- Krebs, N. F. (2014). Food Based Complementary Feeding Strategies for Breastfed Infants: What’s the Evidence that it matters? Nutrition today, 49(6), 271-277. [CrossRef]
- A.O.A.C, (2010). Official Methods of Analysis. Association of Official Analytical Chemists, 18th edition, Washinton D.C., United States of America.
- Olokodona, F. A. (2005). Analysis of Fruit Drinks and Fruit Juices. Institute of Public Analysts of Nigeria (IPAN) Bulletin 6 (24): 9-14.
- Harrigan, W.F. and McCance, M.E. (1976) Laboratory Methods in Food and Dairy Microbiology. Academic Press Inc. Limited, London.
- Ihekoronye, A. I. and Ngoddy, P. O. (1985). Integrated Food Science and Technology for the Tropics, Macmillian Publishers Limited, London, 236 – 253.
- Torres, A., Frias, J., Granito, M. and Vidal-Valverde, C. (2006) Fermented Pigeon Pea (Cajanus cajan) Ingredients in Pasta Products. Journal of Agriculture and Food Chemistry, 54(18): 6685-6691. [CrossRef]
- Reyes-Bastidas, M., Reyes-Fernández, E. Z., López-Cervantes, J., Milán-Carrillo, J. and Loarca-Piña, G. F. (2010). Physicochemical, Nutritional and Antioxidant Properties of Tempeh Flour from Common Bean (Phaseolusvulgaris L.). Food Science and Technology International 16(5): 427-434. [CrossRef]
- Ruiz-Teran, F. and Owens, J. D. (1996). Chemical and Enzymatic Changes during the Fermentation of Bacteria-free Soya Bean Tempe. Journal of the science of Food agriculture, 71: 523-530. [CrossRef]
- Nout, M. J. R., Kiers, J. L. (2005). Tempeh Fermentation, Innovation and Functionality: Update into the third millinium. Journal of Applied Microbiology, 98(4): 789-805. [CrossRef]
- Rubina, N., Muhammad, N., Muhammad, I. and Quratulain, S. (2018). Nutritional Enhancement of Barley in Solid state Fermentation by RhizopusOligosporus ML-10. Nutrition and Food science International Journal, 6(5): 555700. [CrossRef]
- Sefa – Dedeh, S. and Kluvitse, Y. M. (1995). Development of Cowpea – Fortified Weaning Foods: Functional and Chemical properties. Paper Presentation at the Annual Meeting of the Institute of Food Technologists, Atlanta, Georgia, 3-7 June 1995.
- Sanni, A. I., Onilude, A. A. and Ibidapo, O. F. (1999). Physicochemical Characteristics of Weaning Food Formulated from Different Blends of Cereal and Soybean. ZlebensmuntersForsch A 208: 221-224. [CrossRef]
- Cuevas-Rodrı́guez, E. O., MiIán-Carrillo, J., Mora-Escobedo, R., Cárdenas-Valenzuela, O. G. and Reyes-Moreno, C. (2004). Quality Protein Maize (Zea mays L.) and Tempeh Flour through Solid State Fermentation Process. LWT Food Science and Technology 37: 59-67. [CrossRef]
- Lena, G. I., Patroni, E. and Quaglia, G. (2008). Improving the nutritional value of wheat bran by a white-rot fungus. International Journal of Food Science and Technology, 32(6):513-519. [CrossRef]
- Origbemisoye, B. A. and Ifesan, B. O. T. (2019). Chemical Compostion of Kiaat (Pteropcarpusangolensis) bark and the effect of herb pastes on the quality changes in marinated cat fish during chilled storage. Food Biology, 82: 7-12. [CrossRef]
- Codex, A. C. (2010). Corn Soya Sugar Blend for Young Children and Adults. Food and Agriculture Organization of the United Nations. Rome.
- Brons, C., Jensen, C. B., Storggard, H., Alibegovic, A., Jacobsen, S., Nilsson, E., Astrup, A., Quistroff, B. and Vaag, A. (2008). Mitochondrial function in skeletal muscle is normal and unrelated to insulin action in young men born with low birth weight. Journal of clinical endocrinology and metabolism, 93(10):3885-3892. [CrossRef]
- Egounlety, M. (2002). Production of Legume-fortified Weaning foods. Food Research International. 35, 233-237. [CrossRef]
- Abidin, P. E. and Amoaful, E. F. (2015). Healthy Eating for Mothers, Babies and Children: Facilitator Guide for use By Community Health workers in Ghana. International potato center (CIP). Sub-saharan Africa (SSA): Nutrition Department of the Ghana Health Service. 16.
- Oboh, G. (2006). Nutrient Enrichment of Cassava Peels Using a Mixed Culture of Saccharomycescerevisae and Lactobacillus spp. Solid Media Fermentation Techniques. Electronic Journal of Biotechnology, 9(1):46-49. [CrossRef]
- Piermaria, J., Mariano L.de la Canal, Abraham, A.G. (2008). Gelling properties of Kefiran, a food-grade polysaccharide obtained from Kefir grain. Food Hydrocolloids, 22(8): 1520-1527. [CrossRef]
- Singh, U. (2001). Functional properties of grain legume flours. Journal of Food Science and Technology, 38: 191-199.
- Adeyeye, E. I., and Aye, P. A. (1998). The Effect of Sample Preparation on Proximate Composition and the Functional Properties of African Yam Bean Flours (SphenostylisstenocarpaHoshst ex A. rich) Flours. The Italian review of fatty substances, 75(5): 253-261.
- Chandrasekar. V., Ganapathy, S. and Karthikeyan, S. (2016a). Enhancing Alpha Amylase Activity of Finger Millet (Eluesinecoracana) for Improving Baking Property through Solid State Fermentation. Advances in life sciences 5(10): 4069-4076.
- John, L. (2008). Why Iron is Important in Infant Development. The Journal of Nutrition, 138 (12), 2534-2536. [CrossRef]
- USDA. (2012). Nutrition and your health: dietary guidelines for Americans. US Department of Agriculture. Department of Health and Human Services, Washington, DC.
- Lucretia, L., Patience, C. E. and Enobong, M. (2017). Proximte Composition, Micronutrient and Sensory Properties of Complementary Food Formulated from Fermented Maize, Soybeans and Carrot flours. Sky Journal of Food Science,6 (3), 33-39.
- Waquas, U., and Daniel, W. (2011). Zinc Supplementationin the Management of Diarrhoea. Toronto: World Healh Organization(WHO). https://www.who.int/elena/titles/bbc/zinc_diarrhoea/en/.
- FAO/WHO (1991). Essential Amino Acid and Minerals. In Report of a Joint FAO/WHO Experts Consultations. Food and Agricultural Organizations of the United Nations, Rome, 280.
- Ekweagwu, E., Awu, A. E., Madukwe, E. (2008). The role of micronutrients in children health: A review of the Literature. African Journal of Biotechnology, 7(21): 3804-3810.
- Kolawole, F. L., Balogun, M. A., Sanni-Olayiwola, H. O. and Abdulkadir, S. O. (2017). Physical and chemical characteristics of moringa-fortified orange sweet potato flour for complementary food. Croatian Journal of Food Technology, Biotechnology and Nutrition. 12(1-2): 37-43.
- Oloo, B. O., B. M. J. and Rose, O. B. (2014). Effects of Lactic Acid Fermentation on the Retention of Beta-carotene Content in Orange Fleshed Sweet Potatoes. International Journal of Food Studies, 3:13-33. [CrossRef]
- Centre for Food Safety (2014). Microbiological Guidelines for Food: For ready to eat foods in general and specific food items. Revised edition.
- Kikafuda, J. K., Abenakyo, L. and Lukwago, F. B. (2006). Nutritional and sensory properties of high energy/nutrient dense composite flour porridges from germinated maize and roasted beans for child-weaning in developing countries: a case for Uganda. Ecology of food and nutrition, 45:279-294. [CrossRef]
- Alawode, E. K., Idowu, M. A., Adeola, A. A., Oke, E. K. and Omoniyi, S. A. (2017). Some quality attributes of complementary food produced from flour blends of orange flesh sweet potato, sorghum, and soybean. Croatian Journal of Food Science and Technology, 9(2), 122-129. [CrossRef]
- Osman, M. A. (2007). Changes in Nutrient Composition, Trypsin inhibitor, Phytate, Tannins and Protein Digestibility of DolichosLablab Seeds (lablabpurpureus (L) sweet) occurring during Germination. Journal of food technology, 5, 294-299. https://medwelljournals.com/abstract/?doi=jftech.2007.294.299.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).