Submitted:
17 January 2023
Posted:
18 January 2023
You are already at the latest version
Abstract
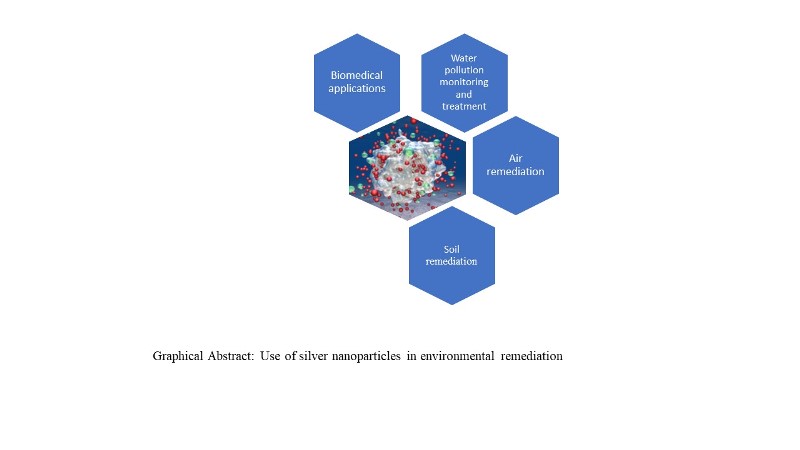
Keywords:
1. Introduction
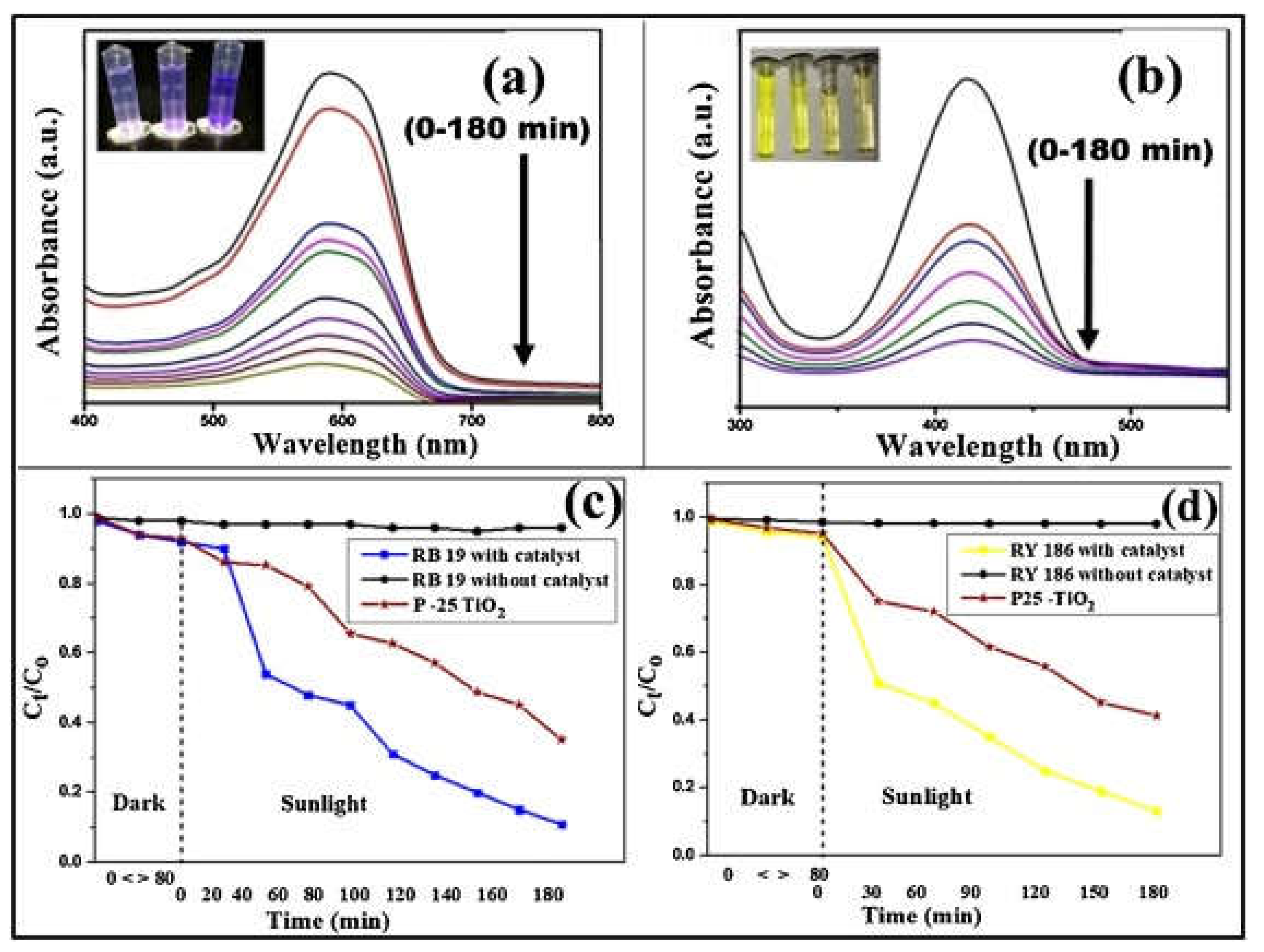
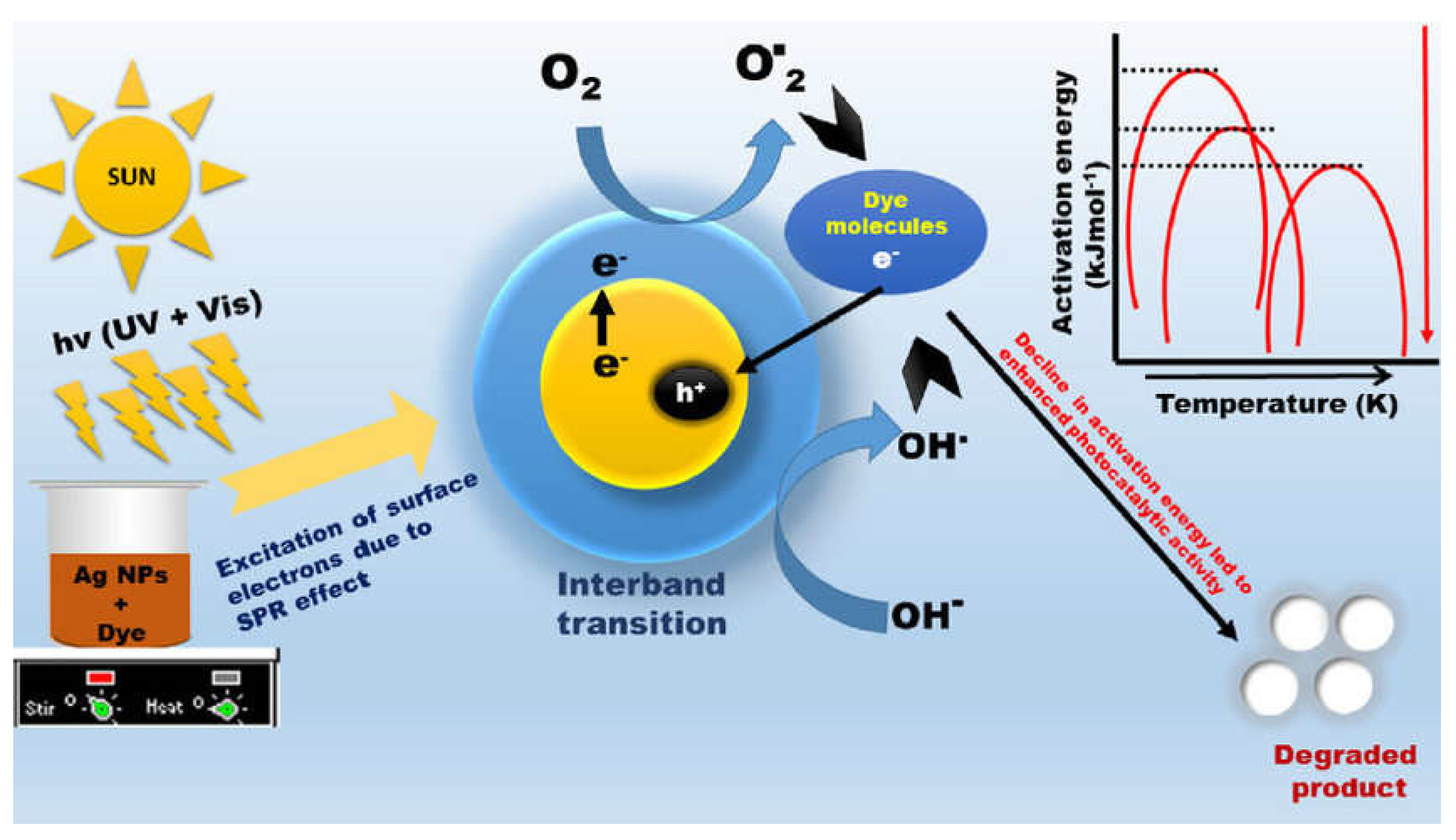
2. Application of Silver Nanoparticles for the Environment
3. Conclusion and Future Perspective
Acknowledgments
Conflicts of Interest
References
- Kodavanti, P. Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose-Response 2005, 3, 273–305. [CrossRef]
- Michael-Kordatou, I.; Michael, C.; Duan, X.; He, X.; Dionysiou, D.D.; Mills, M.A.; Fatta-Kassinos, D. Dissolved effluent organic matter: Characteristics and potential implications in wastewater treatment and reuse applications. Water Res. 2015, 77, 213–248. [CrossRef]
- Fernández, C.; Larrechi, M. S.; Callao, M. P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC - Trends Anal. Chem. 2010, 29, 1202–1211. [CrossRef]
- Carmen, Z.; Daniel, S. Organic pollutants ten years after the stockholm convention-environmental and analytical update, 2012, In Tech.
- Hicks, J. N. Pollutants in our water: Effects on human health and the environment. Otolaryngol. Head. Neck Surg. 1998, 119, 502-505. [CrossRef]
- Graça, M. S. Effects of water pollution on assemblages of aquatic fungi. Limnetica 1994, 10, 41-43. [CrossRef]
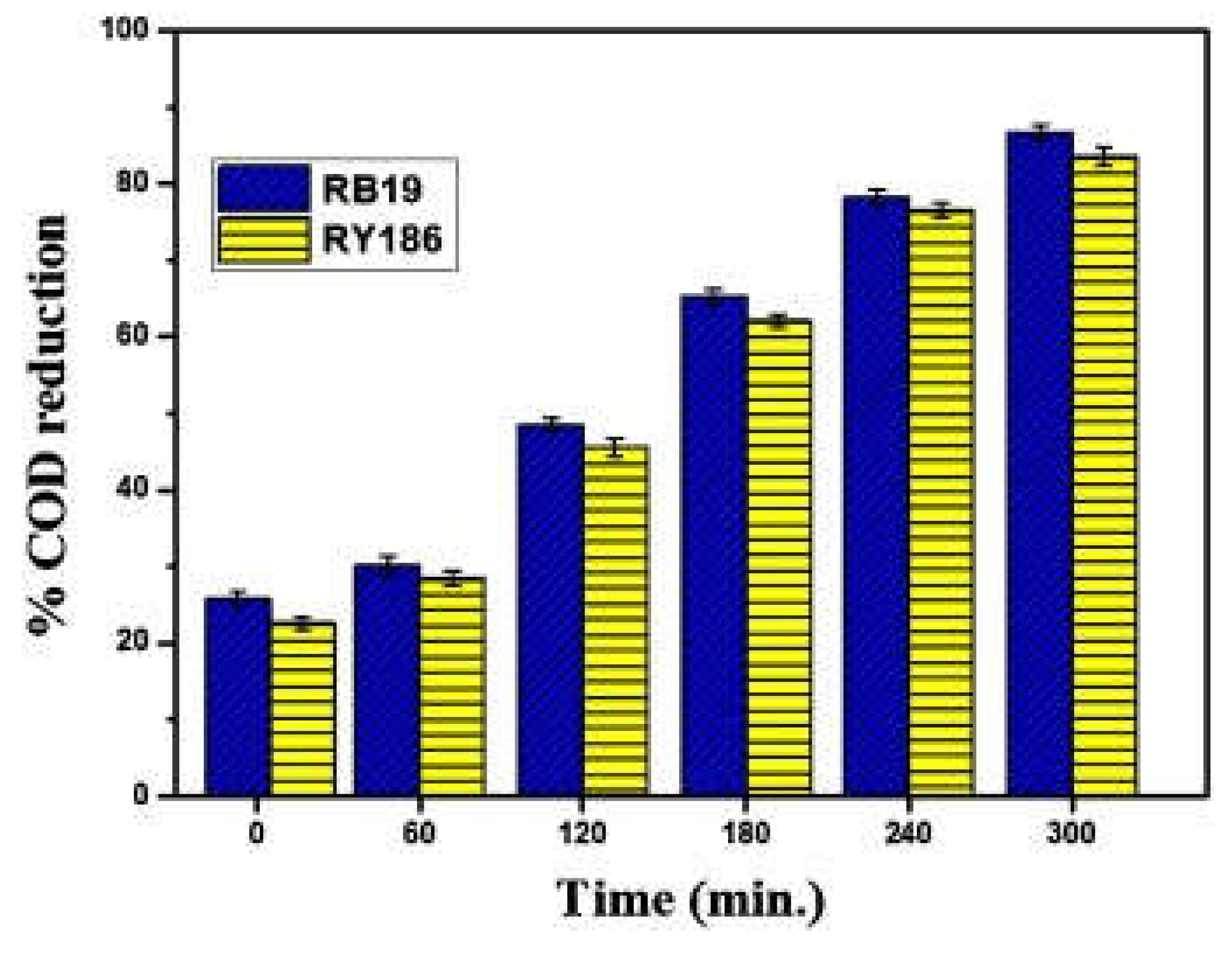
- Khan, M. A. N.; Siddique, M.; Wahid, F.; Khan, R. Removal of reactive blue 19 dye by sono, photo and sonophotocatalytic oxidation using visible light. Ultrason. Sonochem. 2015, 26, 370-377. [CrossRef]
- Pal, K.; Chakroborty, S.; Panda, P.; Nath, N.; Soren, S. Environmental assessment of wastewater management via hybrid nanocomposite matrix implications—an organized review. Environ. Sci. Pollut. Res., 2022, 29, 76626–76643. [CrossRef]
- Panda, P.; Chakroborty, S. Optical sensor technology and its application in detecting environmental effluents: a review. J. Environ. Anal. Chem. 2022, . [CrossRef]
- Nawaz, M. S.; Ahsan, M. Comparison of physico-chemical, advanced oxidation and biological techniques for the textile wastewater treatment. Alexandria Eng. J. 2014, 53, 717-722. [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: a review. Environ. Int. 2004, 30, 953-971. [CrossRef]
- Singh, K.; Arora, S. Removal of Synthetic Textile Dyes from Wastewaters: A Critical Review on Present Treatment Technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807-878. [CrossRef]
- Vandevivere, P.C.; Bianchi, R.; Verstraete, W. Review: Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J. Chem. Technol. Biotechnol. 1998, 72, 289-302.
- Chakroborty, S.; Panda, P. Nanovaccinology against infectious disease. John wiley & sons, inc. Nanovaccinology as targeted therapeutics, 2022, 95-113. [CrossRef]
- Panda, P.; Barik, A.; Unnamatla, M. V.; Chakroborty, S. Synthesis and Antimicrobial Abilities of Metal Oxide Nanoparticles. Springer, Cham, Bio-manufactured Nanomaterials, 2021, 41-58. [CrossRef]
- Agrawal, S.; Bhatt, M.; Rai, S. K.; Bhatt, A.; Dangwal, P.; Agrawal, P. K. Silver nanoparticles and its potential applications: A review. J. pharmacogn. Phytochem. 2018, 7, 930-937.
- Siripattanakul-Ratpukdi, S.; Fürhacker, M. Review: Issues of Silver Nanoparticles in Engineered Environmental Treatment Systems. Water Air Soil Pollut. 2014, 225, 1939. [CrossRef]
- Attatsi, I. K.; Nsiah, F. Application of silver nanoparticles toward Co(II) and Pb(II) ions contaminant removal in groundwater. Appl. Water Sci. 2020, 10, 152. [CrossRef]
- Thangavelu, L.; Veeraragavan, G. R.; Mallineni, S. K.; Devaraj, E.; Parameswari, R. P.; Syed, N. H.; Dua, K.; Chellappan, D. K.; Balusamy, S. R.; Bhawal, U. K. Role of Nanoparticles in Environmental Remediation: An Insight into Heavy Metal Pollution from Dentistry. Bioinorg. Chem. Appl. 2022, 2022, 1946724. [CrossRef]
- Beyene, H. D.; Werkneh, A. A.; Bezabh, H. K.; Ambaye, T. G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18-23. [CrossRef]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and Application of Silver Nanoparticles (AgNPs) for the Prevention of Infection in Healthcare Workers. Int. J. Mol. Sci. 2019, 20, 3620-38. [CrossRef]
- Mo, F.; Zhou, Q.; He, Y. Nano–Ag: Environmental applications and perspectives. Sci. Total Environ. 2022, 829, 154644. [CrossRef]
- Guerra, F. D.; Attia, M. F.; Whitehead, D. C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760-83. [CrossRef]
- Del Prado-Audelo, M. L.; Kerdan, I. G.; Escutia-Guadarrama, L.; Reyna-González, J. M.; Magaña, J. J.; Leyva-Gómez, G. Nanoremediation: Nanomaterials and Nanotechnologies for Environmental Cleanup. Front. Environ. Sci. 2021. [CrossRef]
- Khin, M. M.; Nair, A. S.; Babu, V. J.; Murugana, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075-8109. [CrossRef]
- Ningthoujam, R.; Singh, Y. D.; Babu, P. J.; Tirkey, A.; Pradhan, S.; Sarmae, M. Nanocatalyst in remediating environmental pollutants. Chem. Phys. 2022, 4, 100064. [CrossRef]
- Durgalakshmi, D., Rajendran, S., Naushad, M. Current Role of Nanomaterials in Environmental Remediation. In: Naushad, M., Rajendran, S., Gracia, F. (eds) Advanced Nanostructured Materials for Environmental Remediation. Environmental Chemistry for a Sustainable World. Springer, Cham. 2019, 25. [CrossRef]
- Zoubir, J.; Radaa, C.; Bougdour, N.; Idlahcen, A.; Hayaoui, W. E.; Tajat, N.; MouhriI, W. E.; Nadif, l.; Qourzal, S.; Tamimi, M.; Assabbane, A.; Bakas, I. A sensor based on silver nanoparticles synthesized on carbon graphite sheets for the electrochemical detection of nitrofurazone: Application: Tap water, commercial milk and human urine. J. Indian Chem. Soc. 2022, 99, 100590. [CrossRef]
- Qadri, T.; Khan, S.; Begum, I.; Ahmed, S.; Shah, Z. A.; Ali, I.; Ahmed, F.; Hussain, M.; Hussain, Z.; Rahim, S.; Shah, M. R. Synthesis of phenylbenzotriazole derivative stabilized silver nanoparticles for chromium (III) detection in tap water. J. Mol. Struct. 2022, 1267, 133589. [CrossRef]
- Abdelsattar, A. S.; Gouda, S. M.; Hassana, Y. Y.; Farouka, W. M.; Makky, S.; Nasr, A.; Hakim, T. A.; El-Shibiny, A. In vitro bacteriophage-mediated synthesis of silver nanoparticles for antibacterial applications and heavy metal detection. Mater. Lett. 2022, 318, 132184. [CrossRef]
- Wang, F.; Li, W.; Zhang, W.; Ye, R.; Tan, X. Facile fabrication of the Ag nanoparticles decorated graphitic carbon nitride photocatalyst film for indoor air purification under visible light. Build. Environ. 2022, 222, 109402. [CrossRef]
- Zhao, K.; Ge, L.; Wong, T. I.; Zhou, X.; Lisak, G. Gold-silver nanoparticles modified electrochemical sensor array for simultaneous determination of chromium (III) and chromium (VI) in wastewater samples. Chemosphere, 2021, 281, 130880. [CrossRef]
- Butmee, P.; Samphao, A.; Tumcharern, G. Reduced graphene oxide on silver nanoparticle layers-decorated titanium dioxide nanotube arrays as SERS-based sensor for glyphosate direct detection in environmental water and soil. J. Hazard. Mater. 2022, 437, 129344. [CrossRef]
- Singha, J.; Kumar, V.; Jolly, S. S.; Kim, K.-H.; Rawata, M.; Kukkar, D.; Tsange, Y. F. Biogenic synthesis of silver nanoparticles and its photocatalytic applications for removal of organic pollutants in water. J. Ind. Eng. Chem. 2019, 80, 247–257. [CrossRef]
- Kaur, P.; Thakur, R.; Malwal, H.; Manuja, A.; Chaudhury, A. Biosynthesis of biocompatible and recyclable silver/iron and gold/iron core-shell nanoparticles for water purification technology. Biocatal. Agric. Biotechnol. 2018, 14, 189-197. [CrossRef]
- Ali, Q.; Ahmed, W.; Lal, S.; Sen, T. Novel Multifunctional Carbon Nanotube Containing Silver, and Iron Oxide Nanoparticles for Antimicrobial Applications in Water Treatment. Mater. Today: Proc. 2017, 4, 57–64. [CrossRef]
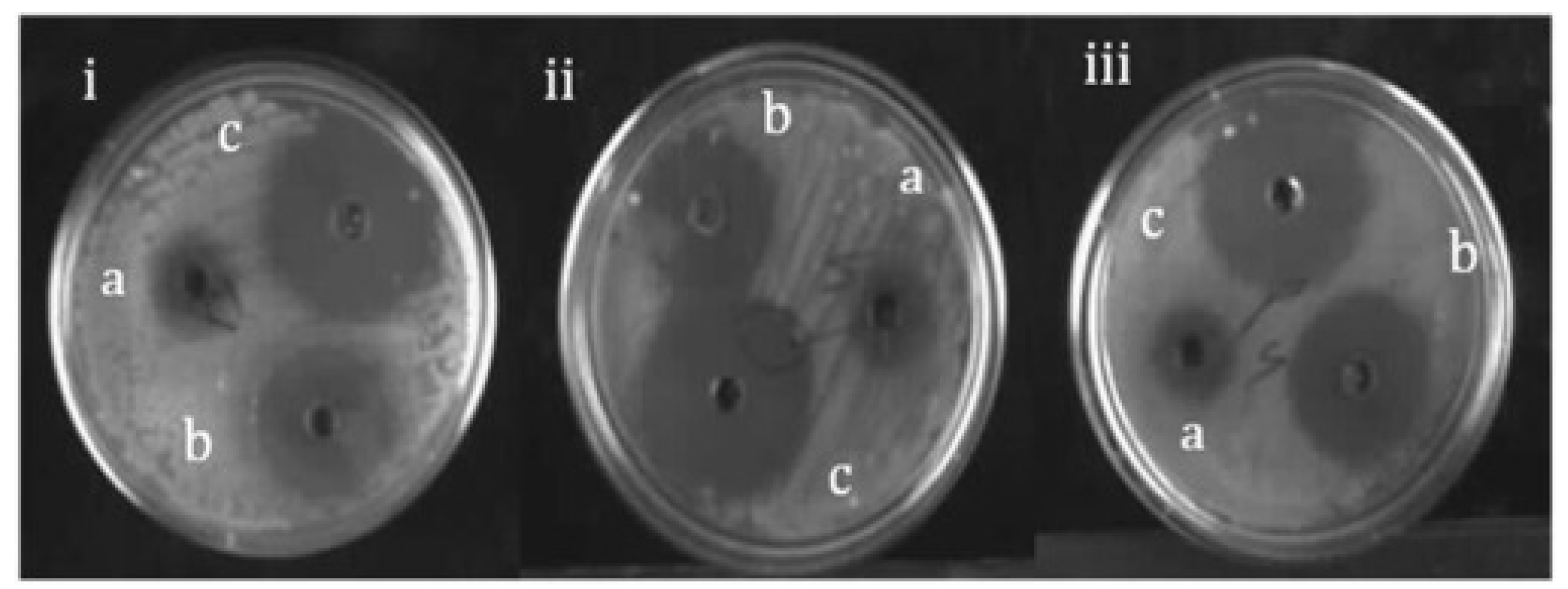
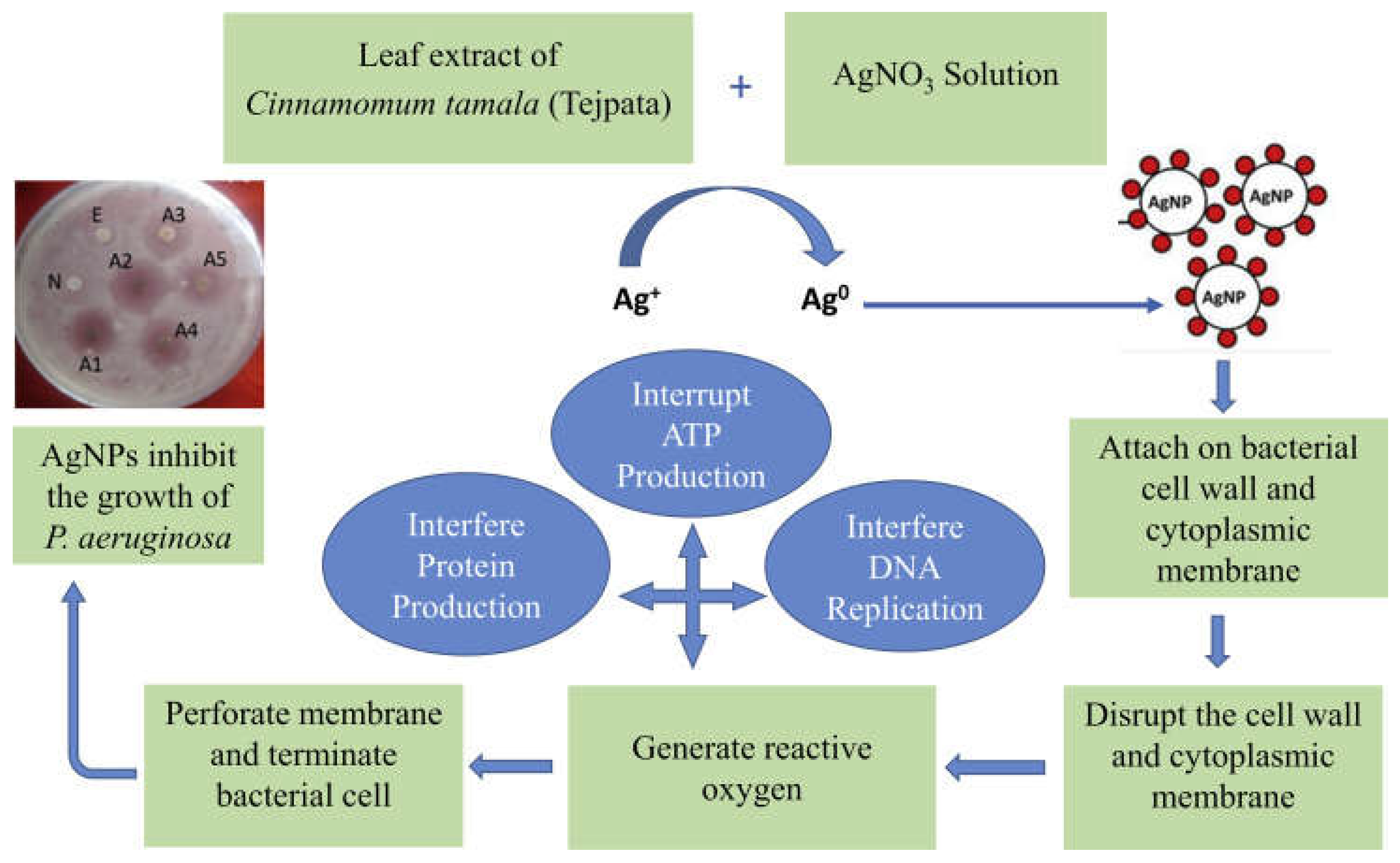
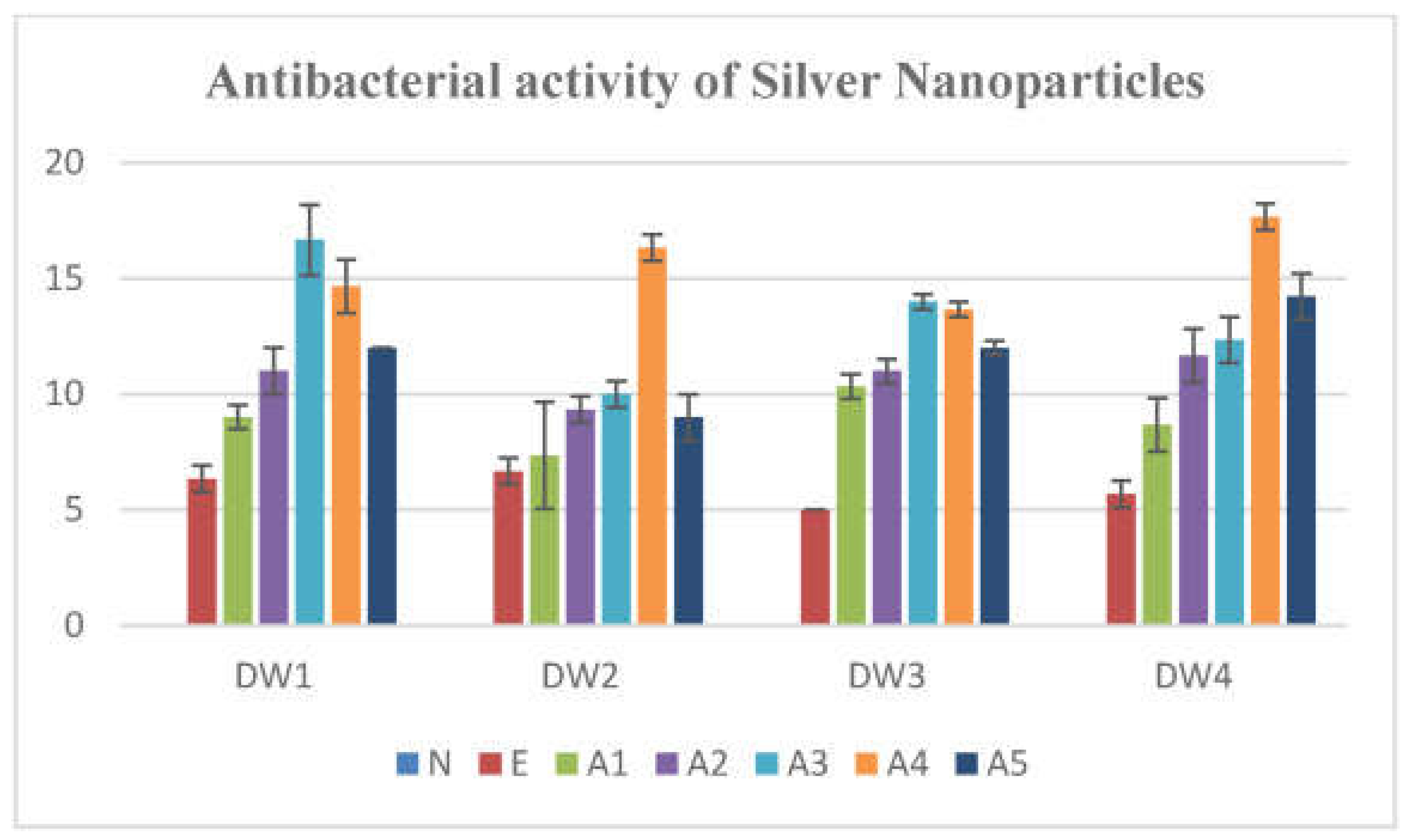
- Mashud, M. A. A.; Moinuzzaman, M.; Hossain, M. S.; Ahmed, S.; Ahsan, G.; Reza, A.; Ratul, R. B. A.; Uddin, M. H.; Momin, M. A.; Jamal, M. A. H. M. Green synthesis of silver nanoparticles using Cinnamomum tamala (Tejpata) leaf and their potential application to control multidrug resistant Pseudomonas aeruginosa isolated from hospital drainage water. Heliyon 2022, 8, e09920. [CrossRef]
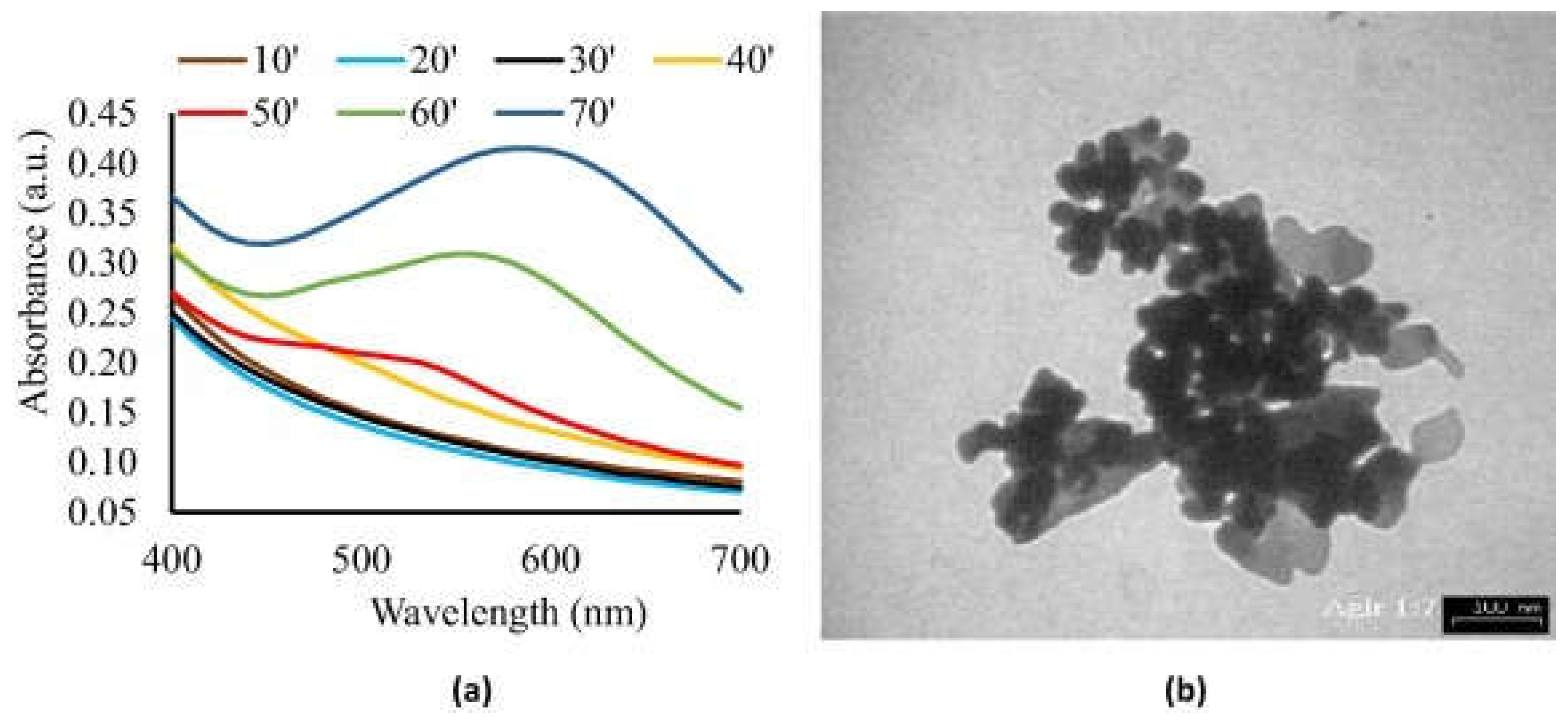
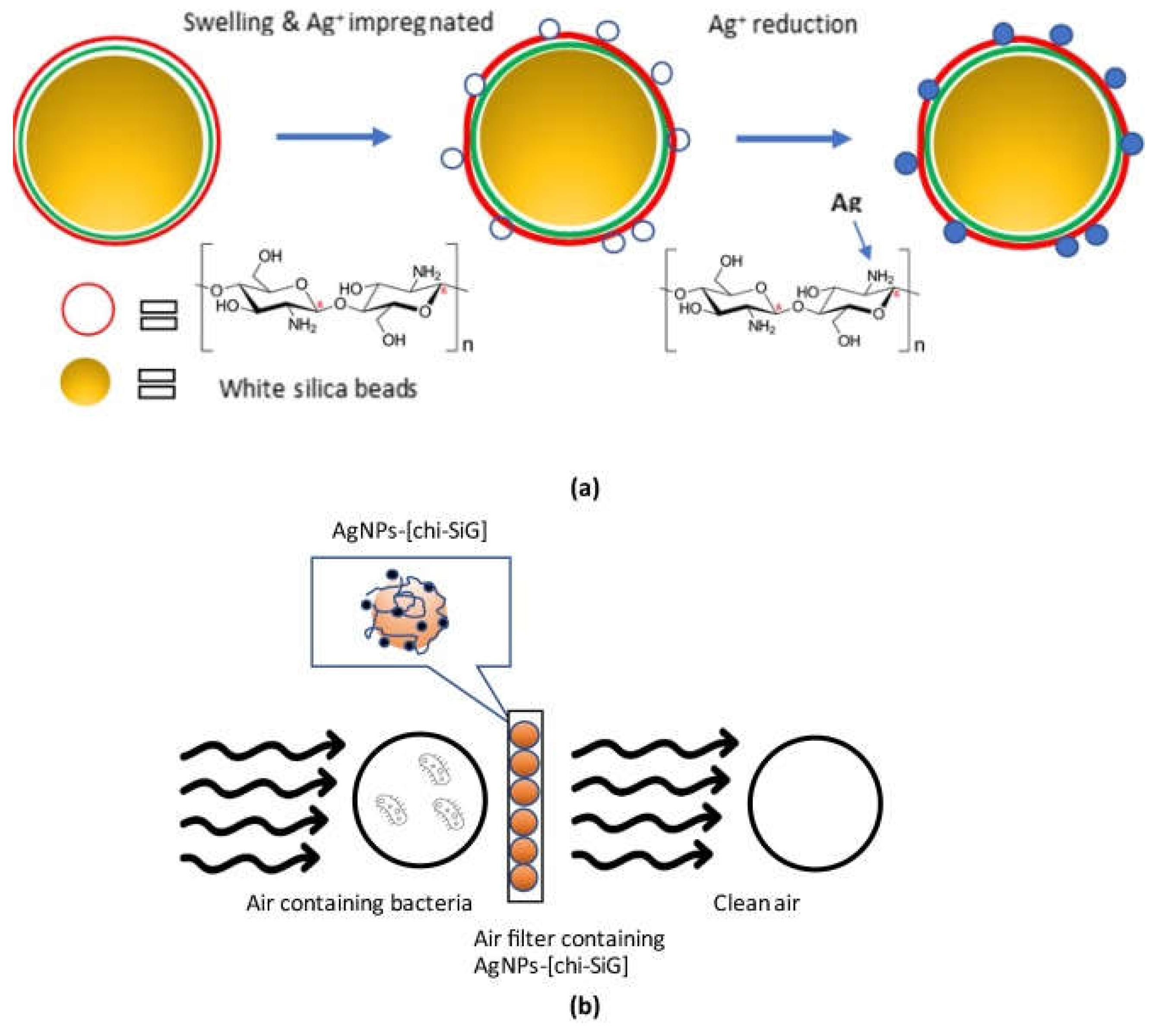
- Hidayat, M. I.; Adlim, M.; Maulana, I.; Suhartono, S.; Hayati, Z.; Bakar, Noor, H. H. A. Green synthesis of chitosan-stabilized silvercolloidal nanoparticles immobilized on white-silicagel beads and the antibacterial activities in a simulated-air-filter. Arab. J. Chem. 2022, 15, 103596. [CrossRef]
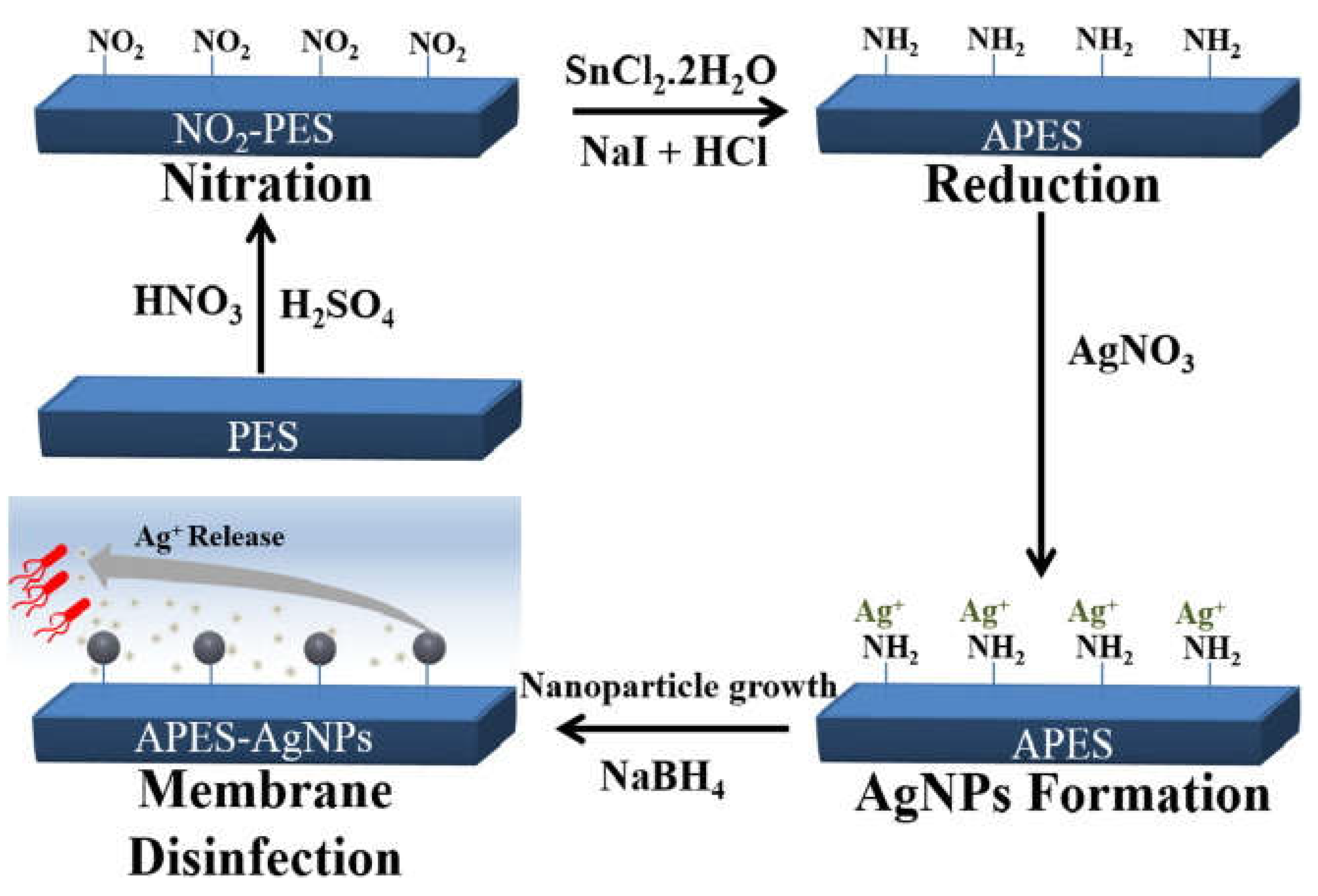
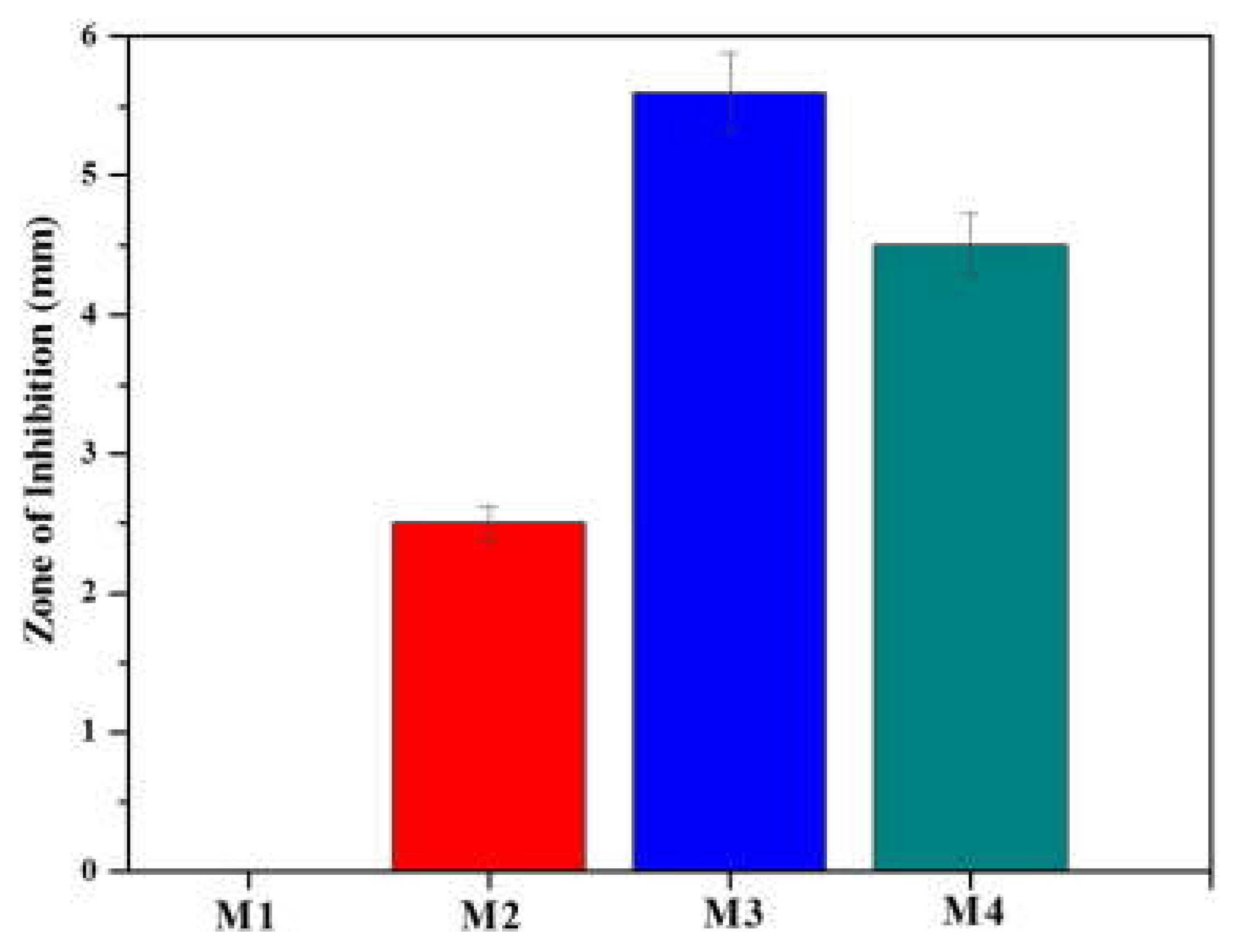
- Haider, M. S.; Shao, G. N.; Imran, S.M.; Park, S. S.; Abbas, N.; Tahir, M. S.; Hussain, M.; Bae, W.; Kim, H. T. Aminated polyethersulfone-silver nanoparticles (AgNPs-APES) compositemembranes with controlled silver ion release for antibacterial and water treatment applications. Mater. Sci. Eng. C 2016, 62, 732–745. [CrossRef]
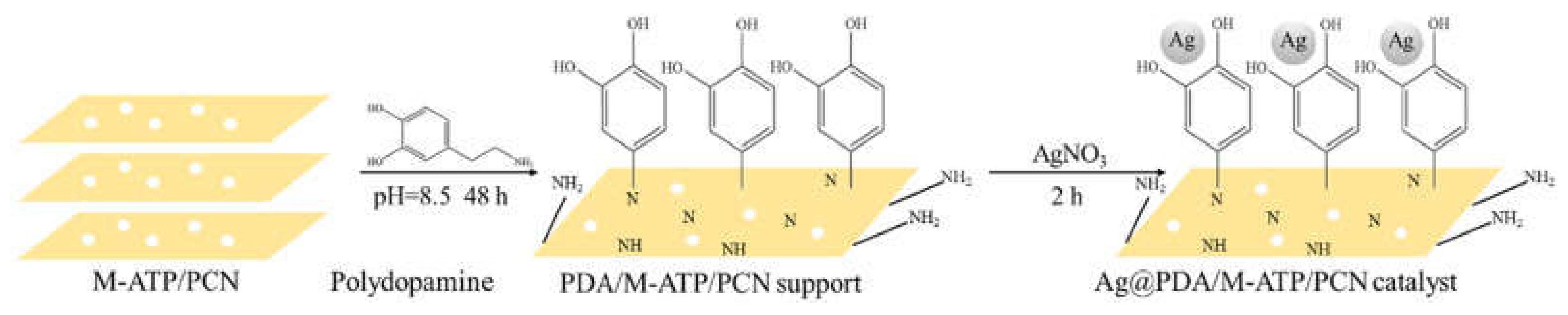
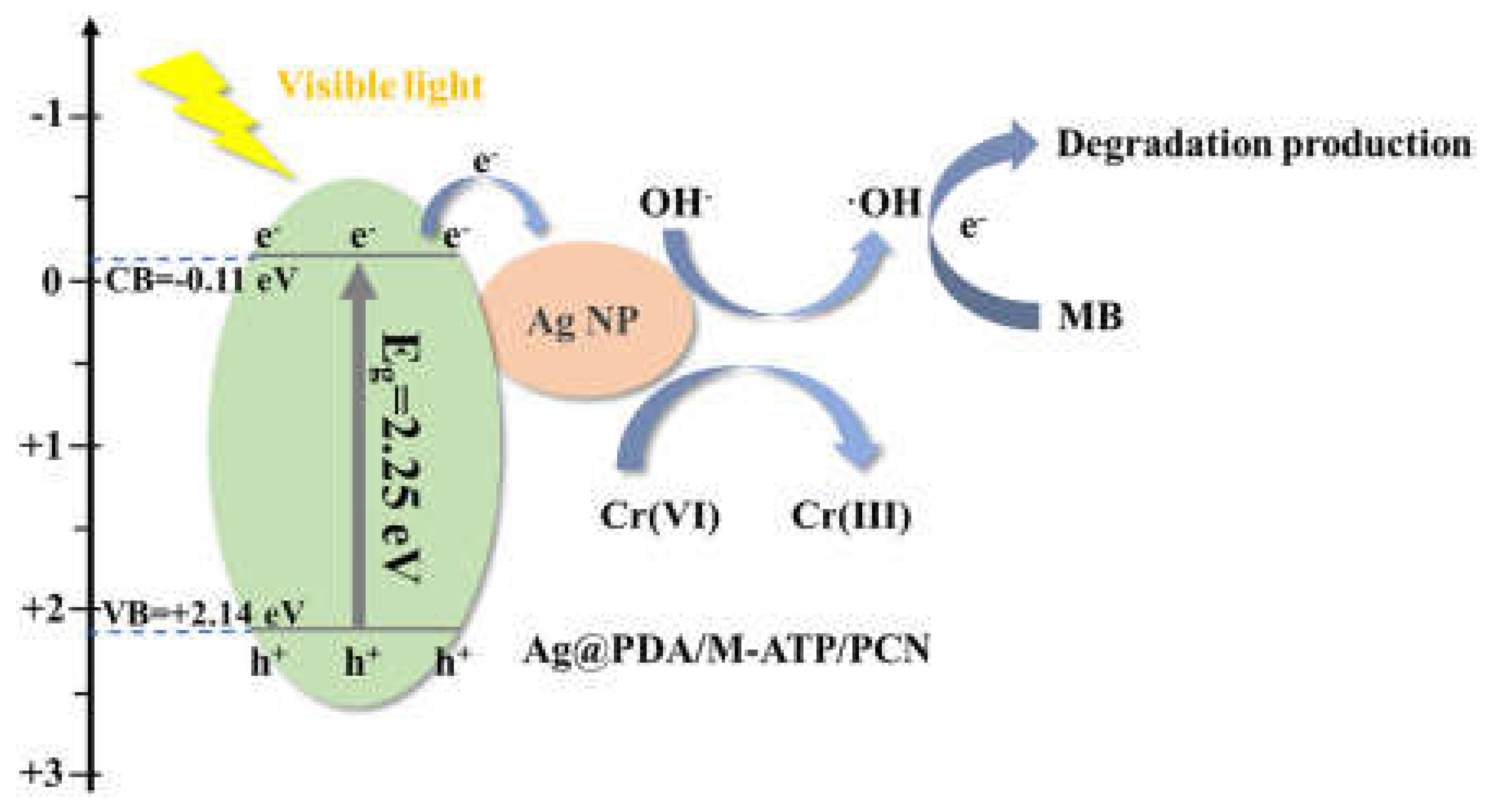
- Zhang, Z.; Zhang, N.; Liu, Y.; Fang, Q.; Xi, J.; Xiao, Y.; Zhou, P.; Xu, L. Efficient degradation of organic dyes and reduced Cr(VI) in environmental water purification by in-situ deposition of silver nanoparticles on polydopamine-modifiedM-ATP/PCN. Catal. Commun. 2022, 172, 106528. [CrossRef]
- Huang, L.; Sun, Y.; Mahmud, S.; Liu, H. Biological and Environmental Applications of Silver Nanoparticles Synthesized Using the Aqueous Extract of Ginkgo biloba Leaf. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1653–1668. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




