Submitted:
19 January 2023
Posted:
24 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
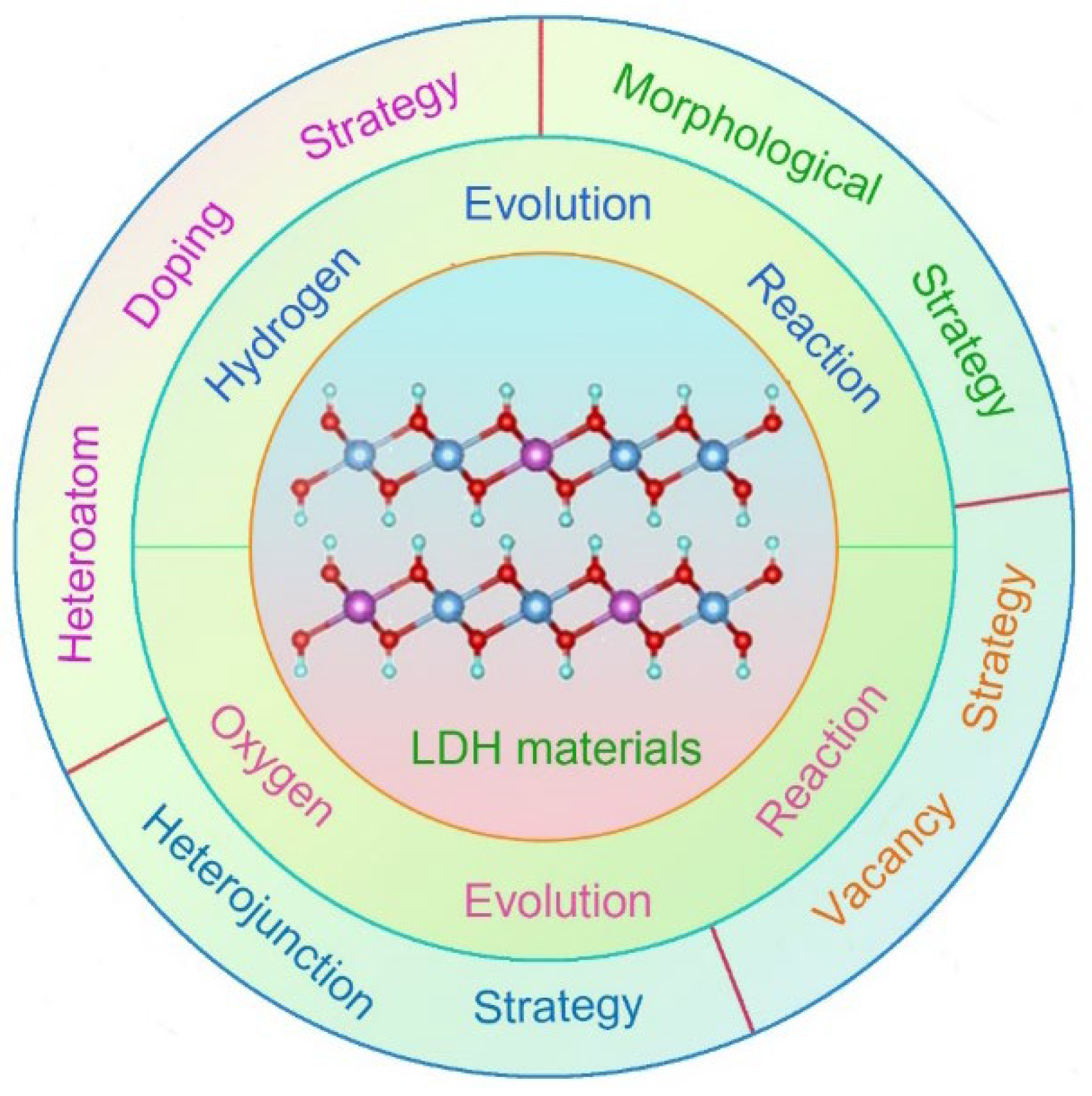
2. Heteroatom doping strategy
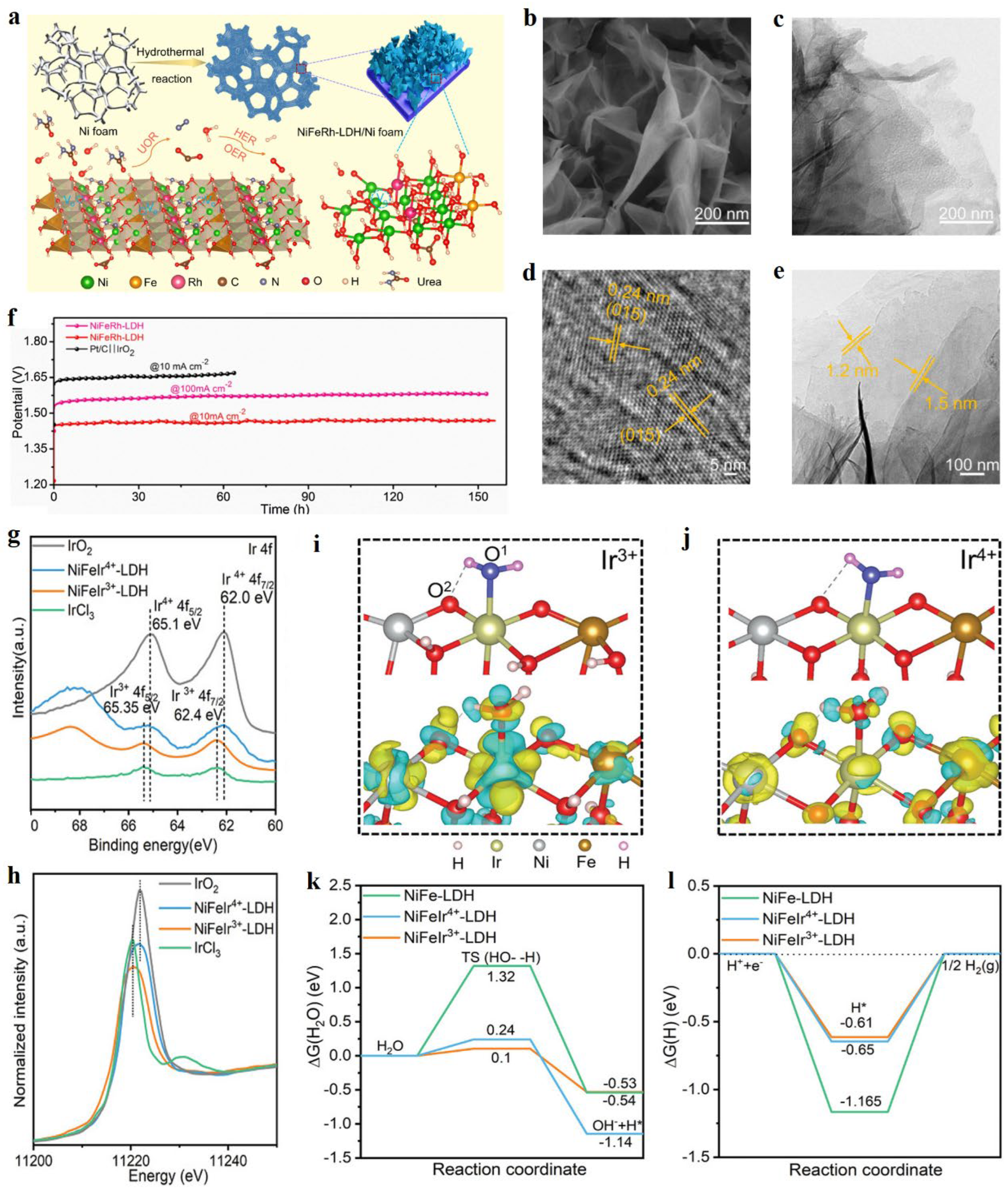
2.1. Precious metal doping
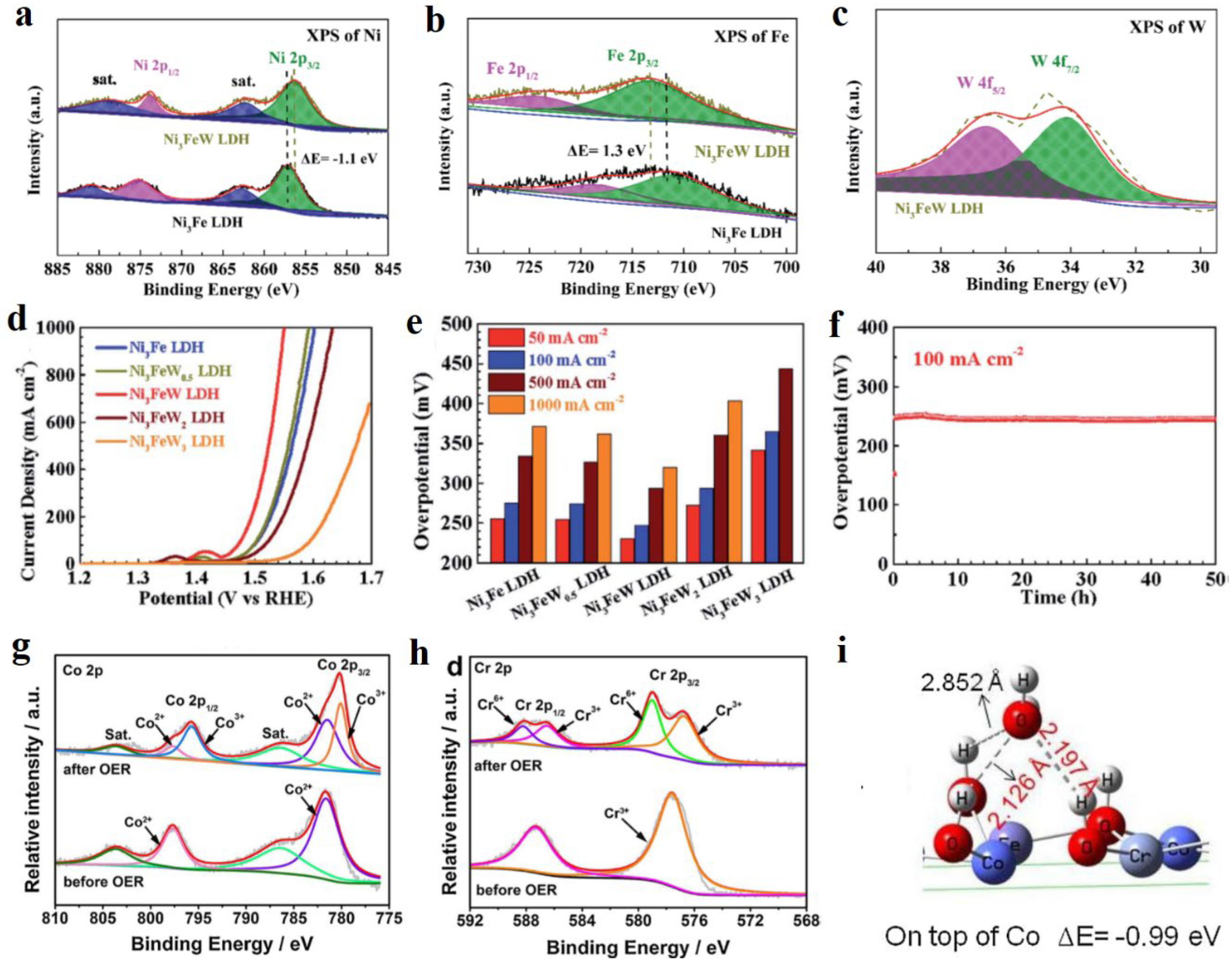
2.2. Non-precious metal doping
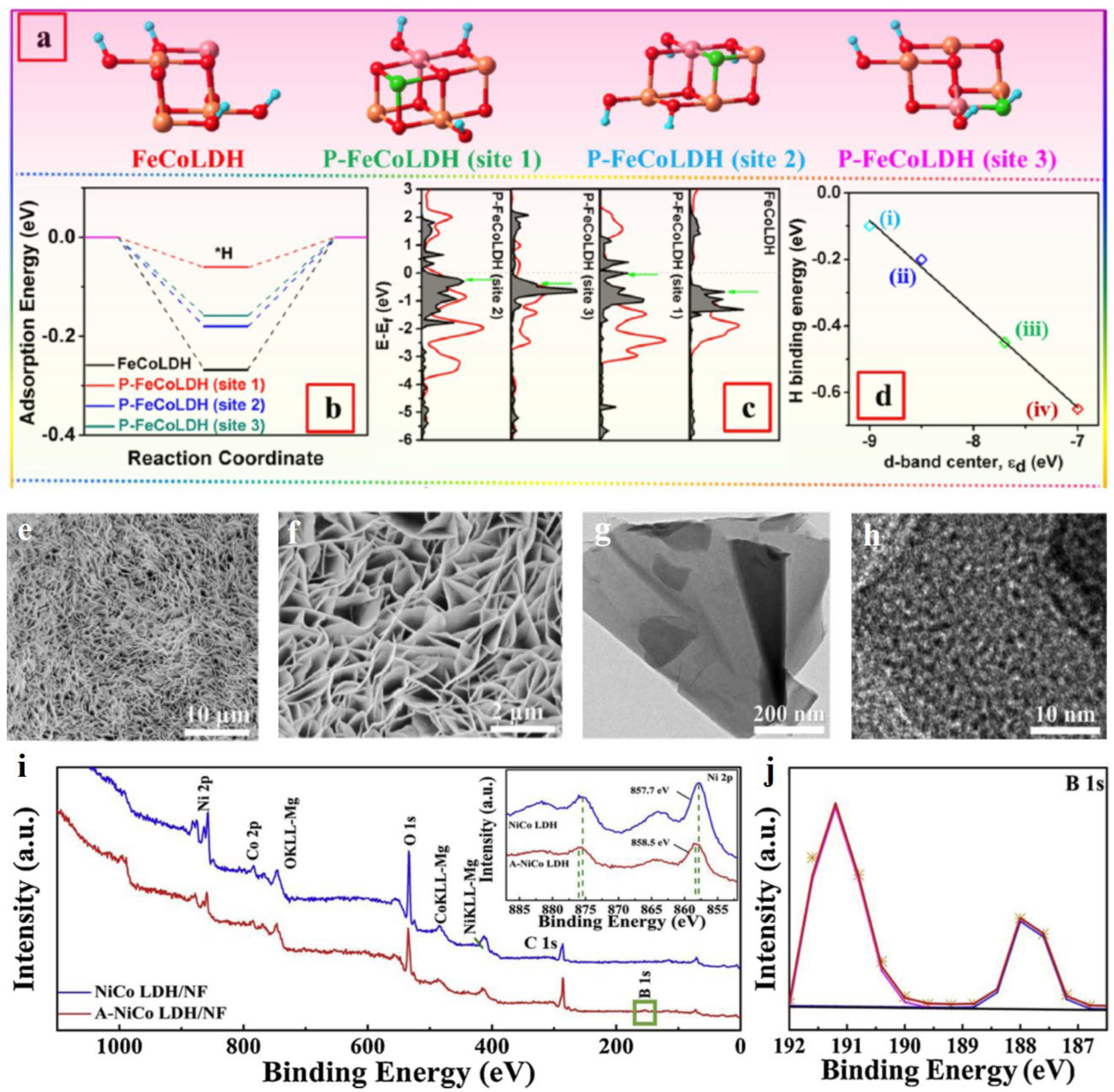
2.3. Non-metal doping
3. Heterojunction strategy
3.1. Metals
3.2. Metal compounds
3.3. Carbon materials
3.4. Organic materials
4. Morphological strategy
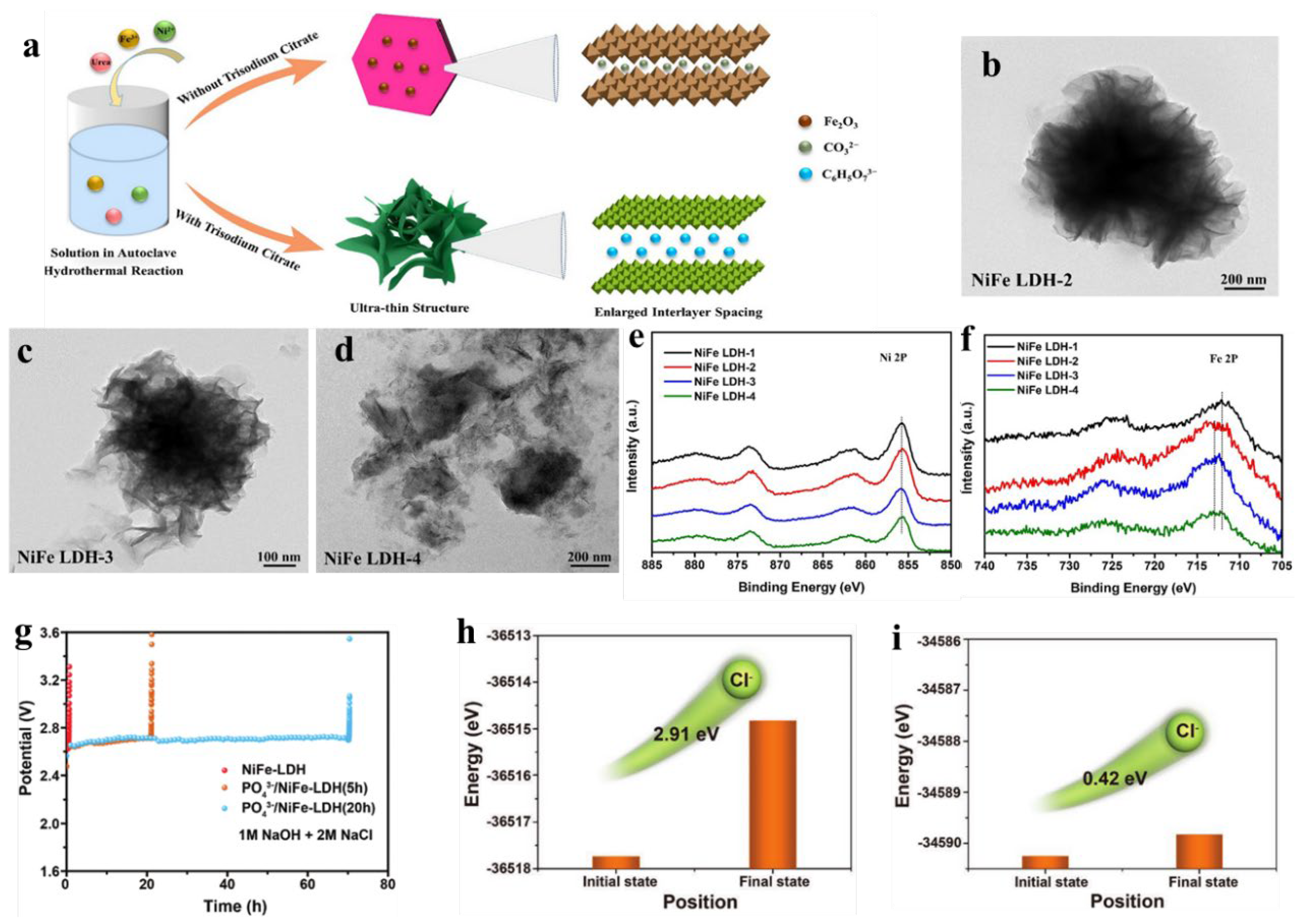
4.1. Intercalation
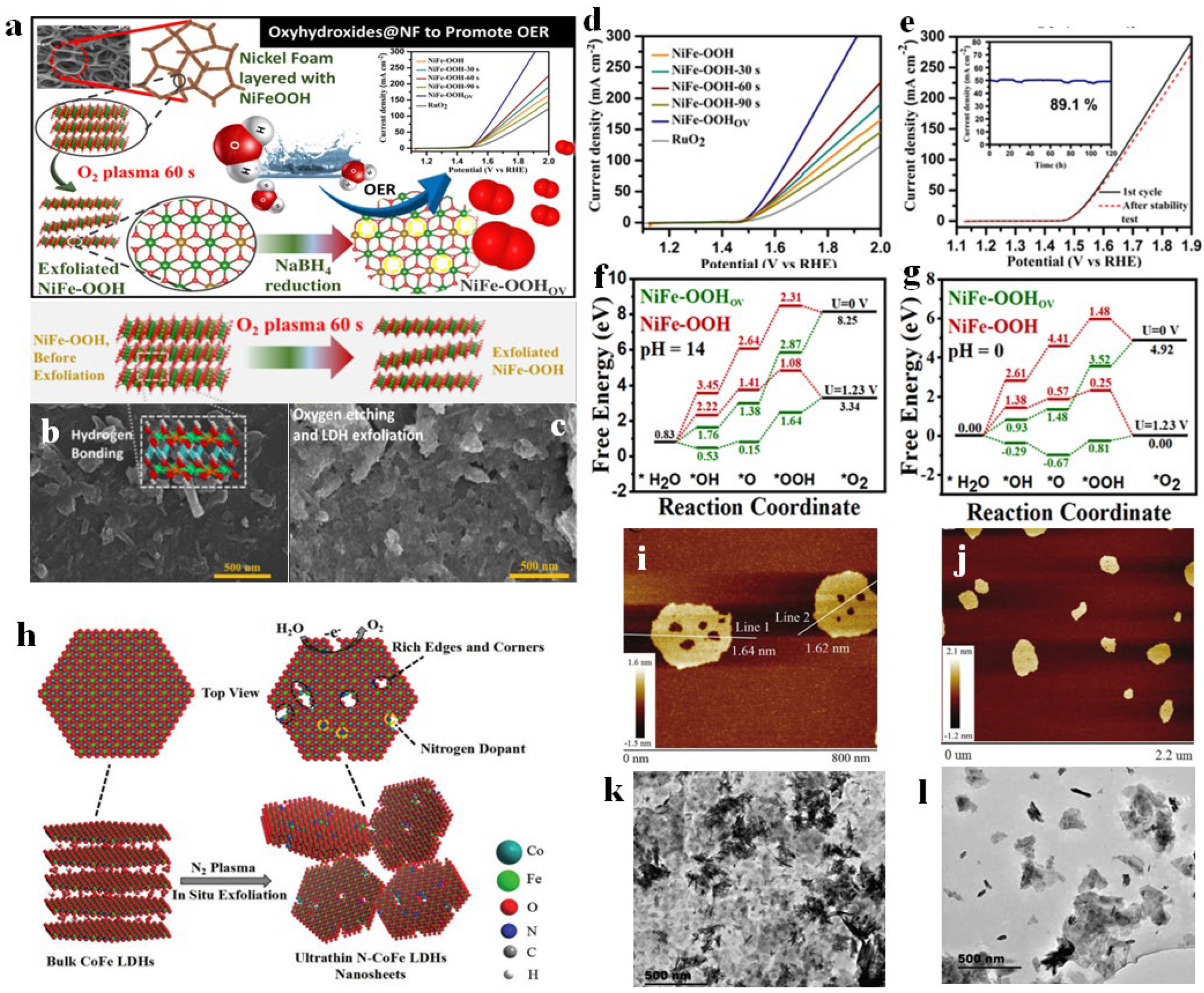
4.2. Exfoliation
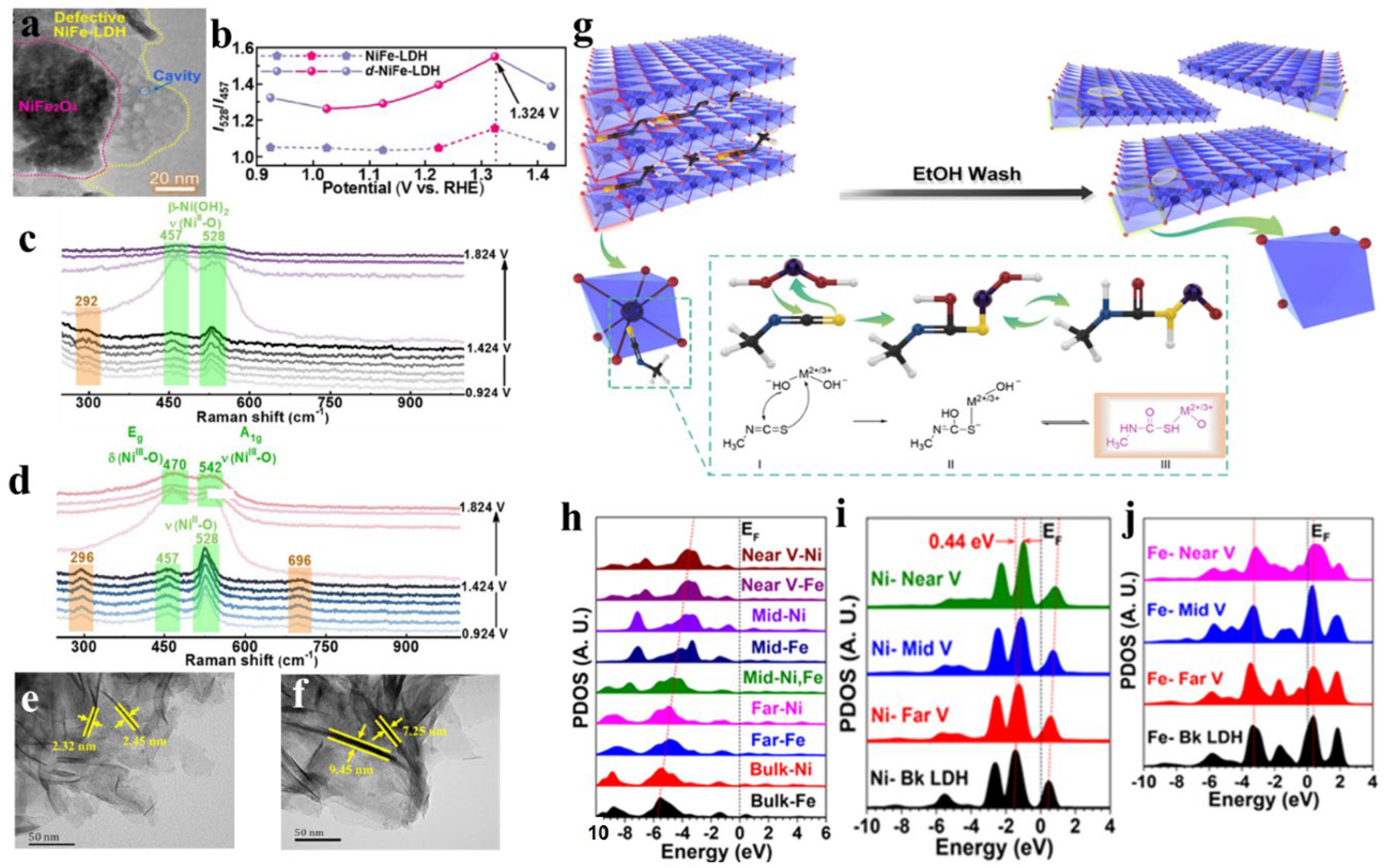
5. Vacancy strategy
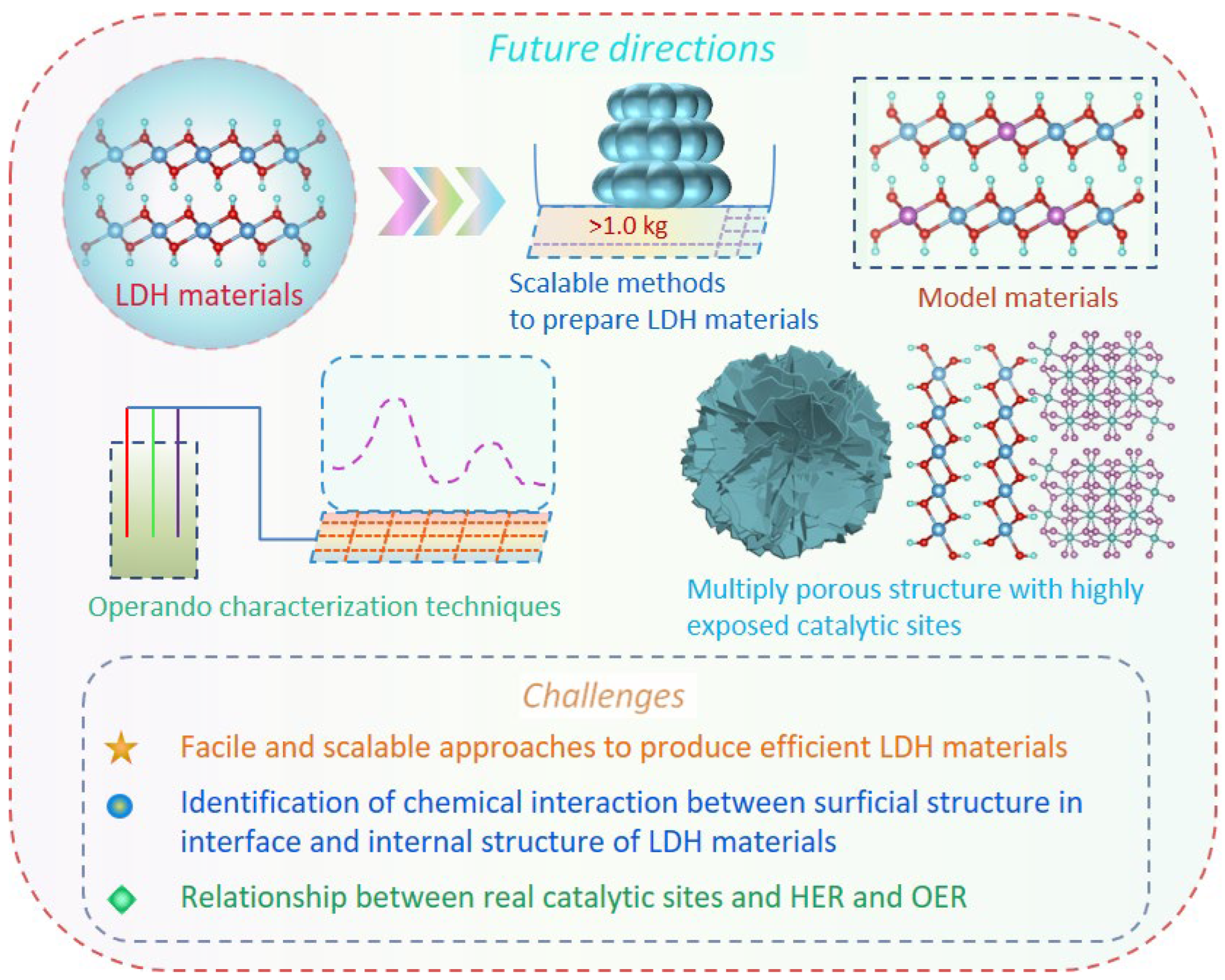
6. Summary and perspective
Acknowledgements
Conflict of Interest
References
- Walter, C.; Menezes, P.M.; Driess, M. Perspective on intermetallics towards efficient electrocatalytic water-splitting. Chem. Sci. 2021, 12, 8603–8631. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, M.F.; Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 2020, 19, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, R.P.; Li, Y.; Yang, P.X.; Wang, D.; Xu, H.; Wang, B.; Meng, F.; Zhang, J.Q.; An, M.Z. ; Electrodeposition: synthesis of advanced transition metal-based catalyst for hydrogen production via electrolysis of water. J. Energy Chem. 2021, 57, 547–566. [Google Scholar] [CrossRef]
- Sun, T.; Mitchell, S.; Li, J.; Lyu, P.; Wu, X.B.; Pérez-Ramírez, J.; Lu, J. Design of local atomic environments in single-atom electrocatalysts for renewable energy conversions. Adv. Mater. 2021, 33, 2003075. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.L.; Zhang, L.; Gong, J.L. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Xiong, B.Y.; Chen, L.S.; Shi, J.L. Anion-containing noble-metal-free bifunctional electrocatalysts for overall water splitting. ACS Catal. 2018, 8, 3688–3707. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Song, B.; Bai, L.C.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X.L. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef]
- Yu, M.Q.; Budiyanto, E.; Tüyüz, H. Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction, Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef]
- Zagalskaya, A.; Alexandrov, V. Role of defects in the interplay between adsorbate evolving and lattice oxygen mechanisms of the oxygen evolution reaction in RuO2 and IrO2. ACS Catal. 2020, 10, 3650–3657. [Google Scholar] [CrossRef]
- Gao, L.K.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z.Q. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8469. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wan, H.; Ma, W.; Zhang, N.; Cao, Y.J.; Liu, X.H.; Wang, J.; Ma, R.Z. Layered metal hydroxides and their derivatives: controllable synthesis, chemical exfoliation, and electrocatalytic applications. Adv. Energy Mater. 2020, 10, 1902535. [Google Scholar] [CrossRef]
- Du, Y.M.; Li, B.; Xu, G.R.; Wang, L. Recent advances in interface engineering strategy for highly-efficient electrocatalytic water splitting. InfoMat 2022. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, S.J.; Zhang, L.C.; Luo, Y.S.; Yang, Q.; Dong, K.; Fang, X.D.; Zheng, D.D.; Alshehri, A.A.; Sun, X.P. N, O-doped carbon foam as metal-free electrocatalysts for efficient hydrogen production from seawater. Nano Res. 2022, 15, 8922–8927. [Google Scholar] [CrossRef]
- Fang, X.D.; Wang, X.G.; Ouyang, L.; Zhang, L.C.; Sun, S.J.; Liang, Y.M.; Luo, Y.S.; Zheng, D.D.; Kang, T.R.; Liu, Q.; Huo, F.; Sun, X.P. Amorphous Co-Mo-B film: a high-active electrocatalyst for hydrogen generation in alkaline seawater. Molecules 2022, 27, 7617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Liang, X.; Wang, L.N.; Sun, K.; Wang, Y.N.; Xie, Z.B.; Wu, Q.N.; Bai, X.Y.; Hamdy, M.S.; Chen, H.; Zou, X.X. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Han, H.; Qiu, Y.L.; Zhang, H.; Bi, T.Y.; Yang, Q.; Liu, M.Y.; Zhou, J.; Ji, X.Q. Lattice-disorder layer generation from liquid processing at room temperature with boosted nanointerface exposure toward water splitting. Sustainable Energy Fuels 2022, 6, 3008–3013. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, L.; Yuan, Z.Y. Recent advances in transition metal layered double hydroxide based materials as efficient electrocatalysts. J. Ind. Eng. Chem 2022. [Google Scholar] [CrossRef]
- Peng, W.F.; Deshmukh, A.; Chen, N.; Lv, Z.X.; Zhao, S.J.; Li, J.; Yan, B.M.; Gao, X.; Shang, L.; Gong, Y.T.; Wu, L.L.; Chen, M.Y.; Zhang, T.R.; Gou, H.Y. ; Deciphering the dynamic structure evolution of Fe- and Ni-codoped CoS2 for enhanced water oxidation. ACS Catal. 2022, 12, 3743–3751. [Google Scholar] [CrossRef]
- Lee, J.S.; Kumar, A.; Yang, T.; Liu, X.H.; Jadhav, A.R.; Park, G.H.; Hwang, Y.; Yu, J.M.; Nguyen, C.T.; Liu, Y.; Ajmal, S.; Kim, M.G.; Lee, H. Stabilizing the OOH* intermediate via pre-adsorbed surface oxygen of a single Ru atom-bimetallic alloy for ultralow overpotential oxygen generation. Energy Environ. Sci. 2020, 13, 5152–5164. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Li, L.L.; Banis, M.N.; Li, J.J.; Adair, K.; Sun, Y.P.; Li, R.Y.; Zhao, Z.J.; Gu, M.; Sun, X.L. Single atom surface engineering: a new strategy to boost electrochemical activities of Pt catalysts. Nano Energy 2022, 93, 106813. [Google Scholar] [CrossRef]
- Mu, X.Q.; Gu, X.Y.; Dai, S.P.; Chen, J.B.; Cui, Y.J.; Chen, Q.; Yu, M.; Chen, C.Y.; Liu, S.L.; Mu, S.C. Breaking the symmetry of single-atom catalysts enables an extremely low energy barrier and high stability for large-current-density water splitting. Energy Environ. Sci. 2022, 15, 4048–4057. [Google Scholar] [CrossRef]
- Bodankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Hameed, A.; Batool, M.; Liu, Z.G.; Nadeem, M.A.; Jin, R.C. Layered double hydroxide-derived nanomaterials for efficient electrocatalytic water splitting: recent progress and future perspective. ACS Energy Lett. 2022, 7, 3311–3328. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.J.; Walsh, A.G.; Yu, J.H.; Zhang, P. Single-atom alloy catalysts: structural analysis, electronic properties and catalytic activities. Chem. Soc. Rev. 2021, 50, 569–588. [Google Scholar] [CrossRef]
- Liu, S.S.; Xu, X.F.; Li, J.S. Silver decorated nickel-cobalt (oxy)hydroxides fabricated via surface reconstruction engineering for boosted electrocatalytic oxygen evolution and urea oxidation. Dalton Trans. 2022, 51, 11814–11822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shan, J.W.; Wang, X.Y.; Hu, Y.J.; Li, Y.Y. Ru/Rh cation doping and oxygen-vacancy engineering of FeOOH nanoarrays@Ti3C2Tx MXene heterojunction for highly efficient and stable electrocatalytic oxygen evolution. Small 2022, 18, 2200173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Y.X; Chen, Z.; Li, Z.J.; Ma, T.Y.; Wu, Z.X.; Wang, L. Trifle Pt coupled with NiFe hydroxide synthesized via corrosion engineering to boost the cleavage of water molecule for alkaline water-splitting. Appl. Catal. B-Environ. 2021, 297, 120395. [Google Scholar] [CrossRef]
- Sun, H.C.; Zhang, W.; Li, J.G.; Li, Z.S.; Ao, X.; Xue, K.H.; Ostrikov, K.K.; Tang, J.; Wang, C.D. Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis. Appl. Catal. B-Environ. 2021, 284, 119740. [Google Scholar] [CrossRef]
- Fan, R.L.; Mu, Q.Q.; Wei, Z.H.; Peng, Y.; Shen, M.G. Atomic Ir-doped NiCo layered double hydroxide as a bifunctional electrocatalyst for highly efficient and durable water splitting. J. Mater. Chem. A 2020, 8, 9871–9881. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.Q.; Xi, C.; Cheng, C.Q.; Kuai, C.G.; Zheng, X.L.; Zhang, R.; Xie, Y.M.; Dong, C.K.; Chen, Y.J.; Du, X.W. Valence-state effect of iridium dopant in NiFe(OH)2 catalyst for hydrogen evolution reaction. Small 2021, 17, 2100203. [Google Scholar] [CrossRef]
- Fan, B.B.; Wang, H.Z.; Han, X.P.; Deng, Y.D.; Hu, W.B. Single atoms (Pt, Ir and Rh) anchored on activated NiCo LDH for alkaline hydrogen evolution reaction. Chem. Commun. 2022, 58, 8254–8257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, W.J.; Yang, Y.B.; Guo, P.F.; Zhu, B.; Wang, K.; Wang, W.T.; He, Z.H.; Liu, Z.T. Ru-doped NiFe layered double hydroxide as a highly active electrocatalyst for oxygen evolution reaction. J. Electrochem. Soc. 2022, 169, 024503. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhou, T.N.; Gong, Y.Q. Ir-Doped Co(OH)2 nanosheets as an efficient electrocatalyst for the oxygen evolution reaction. Dalton. Trans. 2022, 51, 8832–8839. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z.; Feng, L.G. A review on advanced FeNi-based catalysts for water splitting reaction. Energ. Fuel. 2020, 34, 13491–13522. [Google Scholar] [CrossRef]
- Karmakar, A.; Karthick, K.; Sankar, S.S.; Kumaravel, S.; Madhu, R.; Kundu, S. A vast exploration of improvising synthetic strategies for enhancing the OER kinetics of LDH structures: a review. J. Mater. Chem. A 2021, 9, 1314–1352. [Google Scholar] [CrossRef]
- Tang, Y.H.; Liu, Q.; Dong, L.; Wu, H.B.; Yu, X.Y. Activating the hydrogen evolution and overall water splitting performance of NiFe LDH by cation doping and plasma reduction. Appl. Catal. B-Environ. 2020, 266, 118627. [Google Scholar] [CrossRef]
- Yan, D.F.; Li, Y.X.; Huo, J.; Chen, R.; Dai, L.M.; Wang, S.Y. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, G.Q.; Xu, D.; Lian, X.; Li, H.X.; Chen, W.; Su, C.L. Defect chemistry in 2D materials for electrocatalysis. Mater. Today Energy 2019, 12, 215–238. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.P.; Zhao, G.Q.; Lin, Y.; Yu, Z.W.; Xu, X.; Wang, X.L.; Liu, H.K.; Sun, W.P.; Dou, S.X. Active-site-enriched iron-doped nickel/cobalt hydroxide nanosheets for enhanced oxygen evolution reaction. ACS Catal. 2018, 8, 5382–5390. [Google Scholar] [CrossRef]
- Wu, L.B.; Yu, L.; Zhang, F.H.; Wang, D.Z.; Luo, D.; Song, S.W.; Yuan, C.Q.; Karim, A.; Chen, S.; Ren, Z.F. Facile synthesis of nanoparticle-stacked tungsten doped nickel iron layered double hydroxide nanosheets for boosting oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 8096–8103. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, W.J.; Hu, Y.M.; Guan, M.L.; Xu, L.; Li, H.P.; Bao, J.; Li, H.M. Cr-doped CoFe layered double hydroxides: Highly efficient and robust bifunctional electrocatalyst for the oxidation of water and urea. Appl. Catal. B-Environ. 2020, 266, 118959. [Google Scholar] [CrossRef]
- Dhandapani, H.N.; Mahendiran, D.; Karmakar, A.; Devi, P.; Nagappan, S.; Madhu, R.; Bera, K.; Murugan, P.; Babu, B.R.; Kundu, S. Boosting of overall water splitting activity by regulating the electron distribution over the active sites of Ce doped NiCo-LDH and atomic level understanding of the catalyst by DFT study. J. Mater. Chem. A 2022, 10, 17488–17500. [Google Scholar] [CrossRef]
- Rathore, D.; Banerjee, A.; Pande, S. Bifunctional tungsten-doped Ni(OH)2/NiOOH nanosheets for overall water splitting in an alkaline medium. ACS Appl. Nano Mater. 2022, 5, 2664–2677. [Google Scholar] [CrossRef]
- Luo, H.; Liang, J.; Zhou, J.L.; Yin, Z.; Zhang, Z.Y.; Liu, X.B. Synergistic coupling of FeOOH with Mo-incorporated NiCo LDH towards enhancing the oxygen evolution reaction. New J. Chem. 2022, 46, 7999–8009. [Google Scholar] [CrossRef]
- Shao, W.K.; Wang, Q.F.; Huang, C.; Zhang, D.H. High valence state metal-ion doped Fe-Ni layered double hydroxides for oxygen evolution electrocatalysts and asymmetric supercapacitors. Mater. Adv. 2022, 3, 1816–1824. [Google Scholar] [CrossRef]
- Wang, X.Y.; Tuo, Y.X.; Zhou, Y.; Wang, D.; Wang, S.T.; Zhang, J. Ta-doping triggered electronic structural engineering and strain effect in NiFe LDH for enhanced water oxidation. Chem. Eng. J. 2021, 403, 126297. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Das, K.K.; Mansingh, S.; Sultana, S.; Parida, K. Recent progress in first row transition metal Layered double hydroxide (LDH) based electrocatalysts towards water splitting: A review with insights on synthesis. Coodin. Chem. Rev. 2022, 469, 214666. [Google Scholar] [CrossRef]
- Wang, H.; Sun, F.M.; Qi, J.; Zhang, D.; Sun, H.L.; Wang, Q.J.; Li, Z.J.; Wu, Y.A.; Hu, Z.L.; Wang, B. Recent progress on layered double hydroxides: comprehensive regulation for enhanced oxygen evolution reaction. Mater. Today Energy 2022, 27, 101036. [Google Scholar] [CrossRef]
- Feng, H.P.; Yu, J.F.; Tang, L.; Wang, J.J.; Dong, H.R.; Ni, T.; Tang, J.; Tang, W.W.; Zhu, X.; Liang, C. Improved hydrogen evolution activity of layered double hydroxide by optimizing the electronic structure. Appl. Catal. B-Environ. 2021, 297, 120478. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chen, Z.L.; Guo, P.F.; Fei, B.; Wu, R.B. B-doping-induced amorphization of LDH for large-current-density hydrogen evolution reaction. Appl. Catal. B-Environ. 2020, 261, 118240. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Yu, W.L.; Cao, Y.N.; Zhao, J.; Dong, B.; Ma, Y.; Wang, F.L.; Fan, R.Y.; Zhou, Y.L.; Chai, Y.M. S-doped nickel-iron hydroxides synthesized by room-temperature electrochemical activation for efficient oxygen evolution. Appl. Catal. B-Environ. 2021, 292, 120150. [Google Scholar] [CrossRef]
- Liu, Z.j.; Dong, C.L. , Huang, Y.C.; Cen, J.J.; Yang, H.T.; Chen, X.B.; Tong, X.; Su, D.; Wang, Y.Y.; Wang, S.Y. Modulating the electronic structure of ultrathin layered double hydroxide nanosheets with fluorine: an efficient electrocatalyst for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 14483–14488. [Google Scholar] [CrossRef]
- Han, M.; Wang, N.; Zhang, B.; Xia, Y.J.; Li, J.; Han, J.R.; Yao, K.L.; Gao, C.C.; He, C.N.; Liu, Y.C.; Wang, Z.M.; Seifitokaldani, A.; Sun, X.H.; Liang, H.Y. High-valent nickel promoted by atomically embedded copper for efficient water oxidation. ACS Catal. 2020, 10, 9725–9734. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, J.H.; Jo, Y.H.; Lee, J.S. Precipitating metal nitrate deposition of amorphous metal oxyhydroxide electrodes containing Ni, Fe, and Co for electrocatalytic water oxidation. ACS Catal. 2019, 9, 9650–9662. [Google Scholar] [CrossRef]
- Zhou, S.Z.; Jang, H.; Qin, Q.; Li, Z.J.; Kim, M.G.; Li, C.; Liu, X.E.; Cho, J. Three-dimensional hierarchical Co(OH)F nanosheet arrays decorated by single-atom Ru for boosting oxygen evolution reaction. Sci. China Mater. 2021, 64, 1408–1417. [Google Scholar] [CrossRef]
- Jiu, H.F.; Wei, H.; Wang, C.L.; Che, S.C.; Guo, Z.X; Han, Y.X.; Xu, Q.W.; Yu, X.Q.; Zhang, L.X. Construction of Co(OH)F/Ni(OH)2@Fe(OH)3 core-shell heterojunction on nickel foam for efficient oxygen evolution. Int. J. Hydrogen Energ. 2022, 47, 33719–33727. [Google Scholar] [CrossRef]
- Wu, L.L.; Zhang, J.J.; Wang, S.H.; Jiang, Q.; Feng, R.H.; Ju, S.H.; Zhang, W.; Song, F. Silver decorated hydroxides electrocatalysts for efficient oxygen evolution reaction. Chem. Eng. J. 2022, 442, 136168. [Google Scholar] [CrossRef]
- Yu, X.Q.; Guo, J.P.; Li, B.; Xu, J.C.; Gao, P.; Hui, K.S.; Hui, K.N.; Shao, H.Y. Sub-nanometer Pt clusters on defective NiFe LDH nanosheets as trifunctional electrocatalysts for water splitting and rechargeable hybrid sodium-air batteries. ACS Appl. Mater. Interfaces 2021, 13, 26891–26903. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.X.; Chen, Z.; Li, Z.J.; Ma, T.Y.; Wu, Z.X.; Wang, L. Trifle Pt coupled with NiFe hydroxide synthesized via corrosion engineering to boost the cleavage of water molecule for alkaline water-splitting. Appl. Catal. B-Environ. 2021, 297, 120395. [Google Scholar] [CrossRef]
- Han, J.X.; Meng, X.Y.; Lu, L.; Wang, Z.L.; Sun, C.W. Triboelectric nanogenerators powered electrodepositing tri-functional electrocatalysts for water splitting and rechargeable zinc-air battery: a case of Pt nanoclusters on NiFe-LDH nanosheets. Nano Energy 2020, 72, 104669. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liu, J.Y.; Xi, L.F.; Yu, Y.F.; Chen, N.; Sun, S.H.; Wang, W.C.; Kathrin, M. Lange.; Zhang, B. Single-atom Au/NiFe layered double hydroxide electrocatalyst: probing the origin of activity for oxygen evolution reaction. J. Am. Chem. Soc. 2018, 140, 3876–3879. [Google Scholar] [CrossRef]
- Liu, S.S.; Xu, X.F.; Li, S.S. Silver decorated nickel-cobalt (oxy)hydroxides fabricated via surface reconstruction engineering for boosted electrocatalytic oxygen evolution and urea oxidation. Dalton Trans. 2022, 51, 11814–11822. [Google Scholar] [CrossRef]
- Yu, X.Q.; Kang, Y.; Wang, S.; Hui, K.S.; Hui, K.N.; Zhao, H.J.; Li, J.D.; Li, B.; Xu, J.C.; Chen, L. Integrating PtNi nanoparticles on NiFe layered double hydroxide nanosheets as a bifunctional catalyst for hybrid sodium-air batteries. J. Mater. Chem. A 2020, 8, 16355–16365. [Google Scholar] [CrossRef]
- Su, H.; Jiang, J.; Li, N.; Gao, Y.Q.; Ge, L. NiCu alloys anchored defect-rich NiFe layered double-hydroxides as efficient electrocatalysts for overall water splitting. Chem. Eng. J. 2022, 446, 137226. [Google Scholar] [CrossRef]
- Wu, S.H.; Liu, S.Q.; Tan, X.H.; Zhang, W.Y.; Ken, C.; Li, Z. Ni3S2-embedded NiFe LDH porous nanosheets with abundant heterointerfaces for high-current water electrolysis. Chem. Eng. J. 2022, 442, 136105. [Google Scholar] [CrossRef]
- Feng, X.T.; Jiao, Q.Z.; Chen, W.X.; Dang, Y.L.; Dai, Z.; Steven, L.S.; Zhang, J.T.; Zhao, Y.; Li, H.S.; Feng, C.H. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B-Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Yuan, J.J.; Huang, B.J.; Lu, Y.C.; Xu, H.; Qiao, Y.F.; Xu, H.Q.; He, G.Y.; Chen, H.Q. One-step construction of FeNi LDH/FeNi2S4 heterojunctions for boosting electrocatalytic oxygen evolution reaction and hybrid capacitive storage. Appl. Surf. Sci. 2023, 610, 155480. [Google Scholar] [CrossRef]
- Shen, X.R.; Li, H.J.; Zhang, Y.Y.; Ma, T.T.; Li, Q.; Jiao, Q.Z.; Zhao, Y.; Li, H.S.; Feng, C.H. Construction dual-regulated NiCo2S4 @Mo-doped CoFe-LDH for oxygen evolution reaction at large current density. Appl. Catal. B-Environ. 2022, 319, 121917. [Google Scholar] [CrossRef]
- Li, Y.Y.; Guo, H.R.; Zhang, Y.; Zhao, J.Y.; Song, R. Hollow Mo-doped NiSx nanoarrays decorated with NiFe layered double-hydroxides for efficient and stable overall water splitting. J. Mater. Chem. A 2022, 10, 18989–18999. [Google Scholar] [CrossRef]
- Yu, H.Z.; Xie, Y.Y.; Deng, L.M.; Huang, H.J.; Song, J.N.; Yu, D.S.; Li, L.L.; Peng, S.J. In situ construction of FeNi2Se4-FeNi LDH heterointerfaces with electron redistribution for enhanced overall water splitting. Inorg. Chem. Front. 2022, 9, 146–154. [Google Scholar] [CrossRef]
- Nie, F.; Li, Z.; Dai, X.P.; Yin, X.L.; Gan, Y.H.; Yang, Z.H.; Wu, B.Q.; Ren, Z.T.; Cao, Y.H.; Song, W.Y. Interfacial electronic modulation on heterostructured NiSe@CoFe LDH nanoarrays for enhancing oxygen evolution reaction and water splitting by facilitating the deprotonation of OH to O. Chem. Eng. J. 2022, 431, 134080. [Google Scholar] [CrossRef]
- Qi, H.Y.; Zhang, P.; Wang, H.Y.; Cui, Y.M.; Liu, X.; She, X.L.; Wen, Y.H.; Zhan, T.R. Cu2Se nanowires shelled with NiFe layered double hydroxide nanosheets for overall water-splitting. J. Colloid Interf. Sci. 2021, 599, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.Y.; Tang, Z.; Yaseen, M.; Zhan, Y.Z. NiSe and Fe-based layerd double hydroxide nanosheet/Ni foam bifunctional catalyst for water splitting. ACS Appl. Nano Mater. 2022, 5, 16793–16803. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Jiao, L.F. Multifunctional transition metal-based phosphides in energy-related electrocatalysis. Adv. Energy Mater. 2020, 10, 1902104. [Google Scholar] [CrossRef]
- He, K.; Tsega, T.T.; Liu, X.; Zai, J.T.; Li, X.H.; Liu, X.J.; Li, W.H.; Ali, N.; Qian, X.F. Utilizing the space-charge region of the FeNi-LDH/CoP p-n iunction to promote performance in oxygen evolution electrocatalysis. Angew Chem. Int. Ed. 2019, 58, 11903–11909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Yu, H.P.; Yang, J.H.; Zhu, X.R.; Hu, M.J.; Yang, J. Heterostructure engineering of the Fe-doped Ni phosphides/Ni sulfide p-p junction for high-efficiency oxygen evolution. J. Alloy. Compoud. 2022, 924, 166613. [Google Scholar] [CrossRef]
- Wang, X.M.; Zong, X.; Liu, B.; Long, G.F.; Wang, A.Q.; Xu, Z.Q.; Song, R.; Ma, W.G.; Wang, H.; Li, C. Boosting electrochemical water oxidation on NiFe (oxy) hydroxides by constructing schottky junction toward water electrolysis under industrial conditions. Small 2022, 18, 2105544. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qi, J.; Chen, X.; Li, C.; Li, W.P.; Wang, T.H.; Liang, C.H. Three-dimensional heterostructured NiCoP@NiMn-layered double hydroxide arrays supported on Ni foam as a bifunctional electrocatalyst for overall water splitting. ACS Appl. Mater. Interfaces 2020, 12, 4385–4395. [Google Scholar] [CrossRef]
- Gan, Y.H.; Ye, Y.; Dai, X.P.; Yin, X.L.; Cao, Y.H.; Cai, R.; Zhang, X. Self-sacrificial reconstruction of MoO42+ intercalated NiFe LDH/Co2P heterostructures enabling interfacial synergies and oxygen vacancies for triggering oxygen evolution reaction. J. Colloid Interf. Sci. 2023, 629, 896–907. [Google Scholar] [CrossRef]
- Liu, H.J.; Dong, B. Recent advances and prospects of MXene-based materials for electrocatalysis and energy storage. Mater. Today Phys. 2021, 20, 100469. [Google Scholar] [CrossRef]
- Qiao, J.Y.; Kong, L.Q.; Xu, S.K.; Lin, K.X.; He, W.; Ni, M.; Ruan, Q.S.; Zhang, P.G.; Liu, Y.; Zhang, W.; Pan, L.; Sun, Z.M. Research progress of MXene-based catalysts for electrochemical water-splitting and metal-air batteries. Energy Storage Mater. 2021, 43, 509–530. [Google Scholar] [CrossRef]
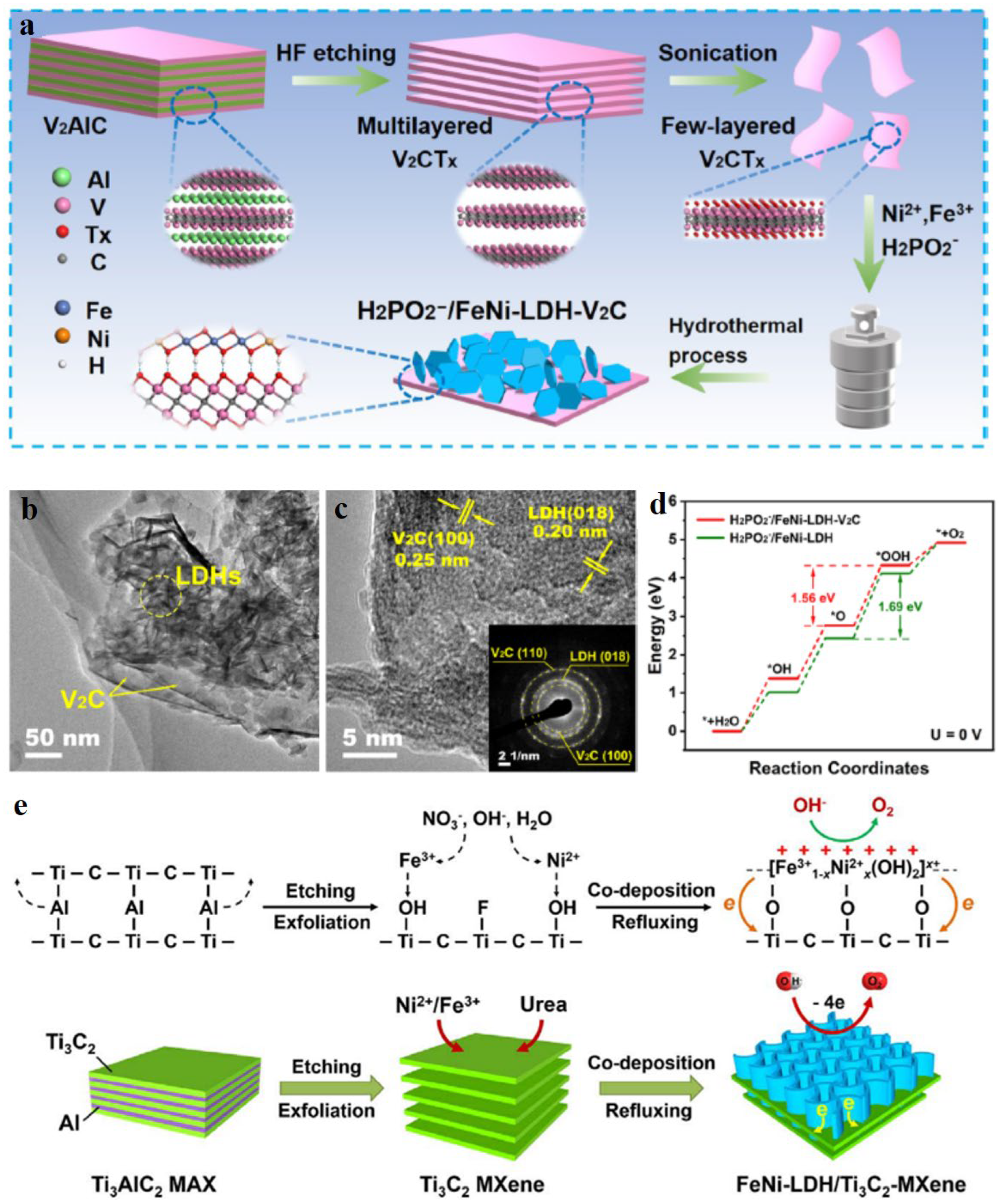
- Chen, Y.F.; Yao, H.L.; Kong, F.T.; Tian, H.; Meng, G.; Wang, S.Z.; Mao, X.P.; Cui, X.Z.; Hou, X.M.; Shi, J.L. V2C MXene synergistically coupling FeNi LDH nanosheets for boosting oxygen evolution reaction. Appl. Catal. B-Environ. 2021, 297, 120474. [Google Scholar] [CrossRef]
- Yu, M.Z.; Zhou, S.; Wang, Z.Y.; Zhao, J.J.; Qiu, J.S. Boosting oxygen evolution by synergistically coupling layered double hydroxide to MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhong, X.; Wu, X.F.; Wang, Y.; Feng, S.H. Optimizing the electronic spin state and delocalized electron of NiCo2(OH)x/MXene composite by interface engineering and plasma boosting oxygen evolution reaction. J. Energy Chem. 2022, 71, 129–140. [Google Scholar] [CrossRef]
- Bai, X.; Guan, J.Q. MXenes for electrocatalysis applications: modification and hybridization. Chinese J. Catal. 2022, 43, 2057–2090. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, X.Y.; Ren, J.W.; Wang, H. NiFe LDH/Ti3C2Tx/nickel foam as a binder-free electrode with enhanced oxygen evolution reaction performance. Int. J. Hydrogen Energ. 2022, 47, 3886–3892. [Google Scholar] [CrossRef]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhang, J.H.; Yu, Q.Y.; Yang, H.Y.; Chen, X.; Yuan, X.; Huang, K.; Xiong, X.L. Synthesis of 3D CoO nanowires supported NiFe layered double hydroxide using an atmospheric pressure microplasma for high-performance oxygen evolution reaction. Chem Eng. J. 2021, 410, 128366. [Google Scholar] [CrossRef]
- Wu, Z.C.; Zou, Z.X.; Huang, J.S.; Gao, F. NiFe2O4 nanoparticles/NiFe layered double-hydroxide nanosheet heterostructure array for efficient overall water splitting at large current densities. ACS Appl. Mater. Interfaces 2018, 10, 26283–26292. [Google Scholar] [CrossRef]
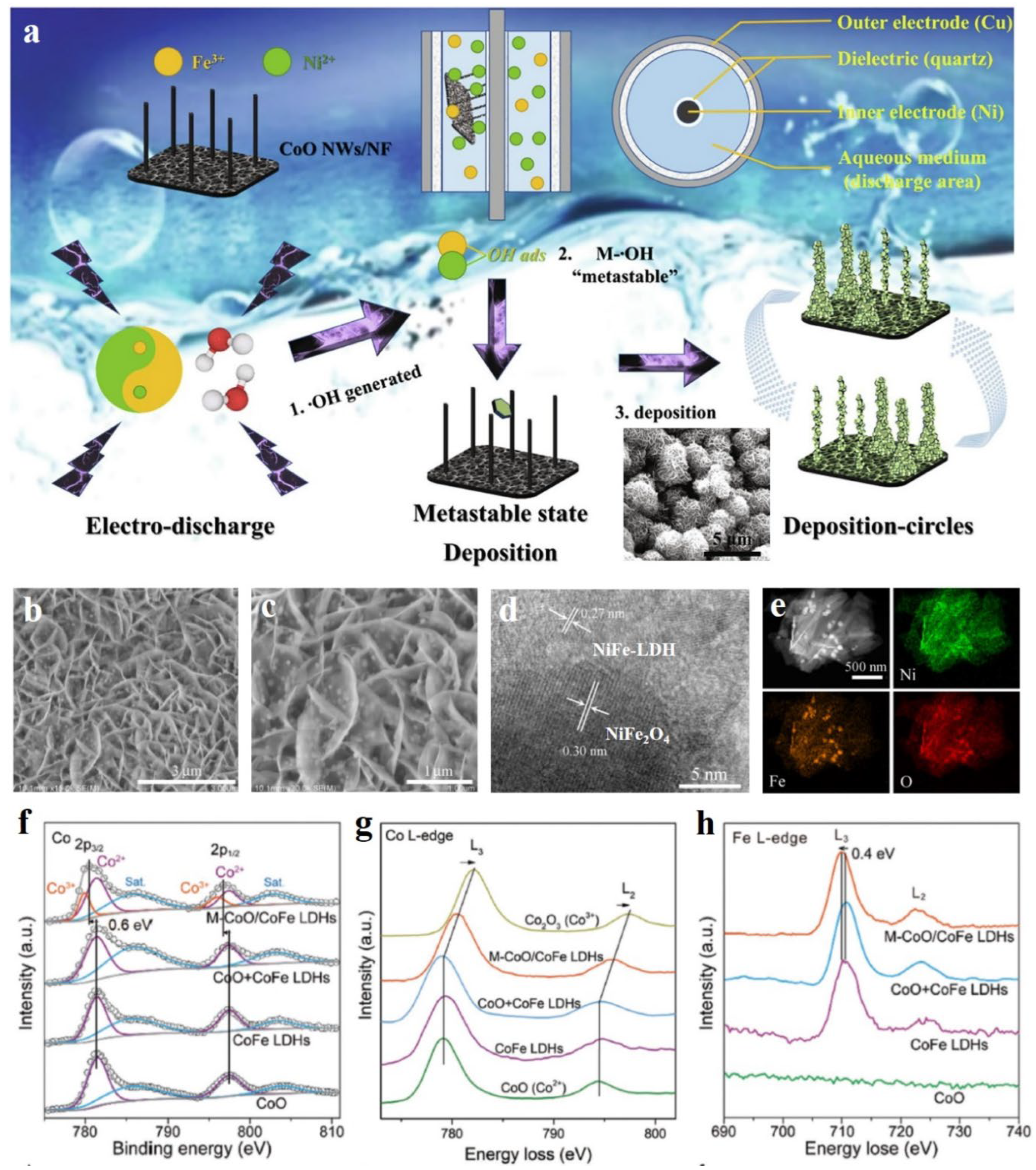
- Gao, Z.W.; Ma, T.; Chen, X.M.; Liu, H.; Cui, L.; Qiao, S.Z.; Yang, J.; Du, X.W. Strongly coupled CoO nanoclusters/CoFe LDHs hybrid as a synergistic catalyst for electrochemical water oxidation. Small, 2018, 14, 1800195. [Google Scholar] [CrossRef]
- Suchada, S.; Li, S.S.; Akihiro, Y.; Li, X.M.; Chanatip, S.; Abuliti, A.; Guan, G.Q. Fabrication of NiO Microflake@NiFe-LDH Nanosheet Heterostructure Electrocatalysts for Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 7, 2327–2334. [Google Scholar]
- Li, D.D.; Li, T.; Hao, G.Y.; Guo, W.J.; Chen, S.; Liu, G.; Li, J.P.; Zhao, Q. IrO2 nanoparticle-decorated single-layer NiFe LDHs nanosheets with oxygen vacancies for the oxygen evolution reaction. Chem. Eng. J. 2020, 399, 125738. [Google Scholar] [CrossRef]
- Hu, J.; Salihy, A.A.; Wang, J.; Li, X.; Fu, Y.F.; Li, Z.H.; Han, X.J.; Song, B.; Xu, P. Improved interface charge transfer and redistribution in CuO-CoOOH p-n heterojunction nanoarray electrocatalyst for enhanced oxygen evolution reaction. Adv. Sci. 2021, 8, 2103314. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, S.C.; Yang, C.H.; Lei, Z.T. Vertically grown p-n heterojunction FeCoNi LDH/CuO arrays with modulated interfacial charges to facilitate the electrocatalytic oxygen evolution reaction. J. Mater. Chem. A 2022, 10, 11938–11947. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, M.S.; Yao, M.Q.; Zhu, J.; Yang, S.D.; Chen, L.; Sun, B.L.; Zhang, J.C.; Hu, W.C.; Zhao, P. Interfacial engineering of an FeOOH@Co3O4 heterojunction for efficient overall water splitting and electrocatalytic urea oxidation. J. Colloid Interf. Sci. 2022, 623, 617–626. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Liu, Z.Q.; Yang, Q.; Zhang, X.Y.; Liu, J.Q.; Liu, M.Y.; Bi, T.Y.; Ji, X.Q. Atmospheric-temperature chain reaction towards ultrathin non-crystal-phase construction for highly efficient water splitting. Chem. Eur. J. 2022, 28, e20220683. [Google Scholar] [CrossRef]
- Sun, T.; Tian, B.B.; Lu, J.; Su, C.L. Recent advances in Fe (or Co)/N/C electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells, J. Mater. Chem. A 2017, 5, 18933–18950. [Google Scholar] [CrossRef]
- Liang, H.Y.; Shi, R.X.; Zhou, Y.; Jiang, W.Y.; Sun, T.; Zhang, Z.Q.; Sun, L.S.; Lian, J.B.; Li, H.M.; Bu, Y.F. Removing cost barriers to template carbon synthesis for high-performance supercapacitors by establishing a zero-emission chemical cycle from CO2. ACS Energy Lett. 2022, 7, 4381–4388. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Qiu, C.T.; Ling, X.; Tian, B.B.; Chen, W.; Su, C.L. B, N codoped and defect-rich nanocarbon material as a metal-free bifunctional electrocatalyst for oxygen reduction and evolution reactions. Adv. Sci. 2018, 5, 1800036. [Google Scholar] [CrossRef]
- Sun, T.; Wu, Q.; Jiang, Y.F; Zhang, Z.Q.; Du, L.Y.; Yang, L.J.; Wang, X.Z.; Hu, Z. Sulfur and nitrogen codoped carbon tubes as bifunctional metal-free electrocatalysts for oxygen reduction and hydrogen evolution in acidic media. Chem. Eur. J. 2016, 22, 10326–10329. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wan, H.; Ma, W.; Zhang, N.; Cao, Y.J.; Liu, X.H.; Wang, J.; Ma, R.Z. Layered metal hydroxides and their derivatives: controllable synthesis, chemical exfoliation, and electrocatalytic applications. Adv. Energy Mater. 2020, 10, 1902535. [Google Scholar] [CrossRef]
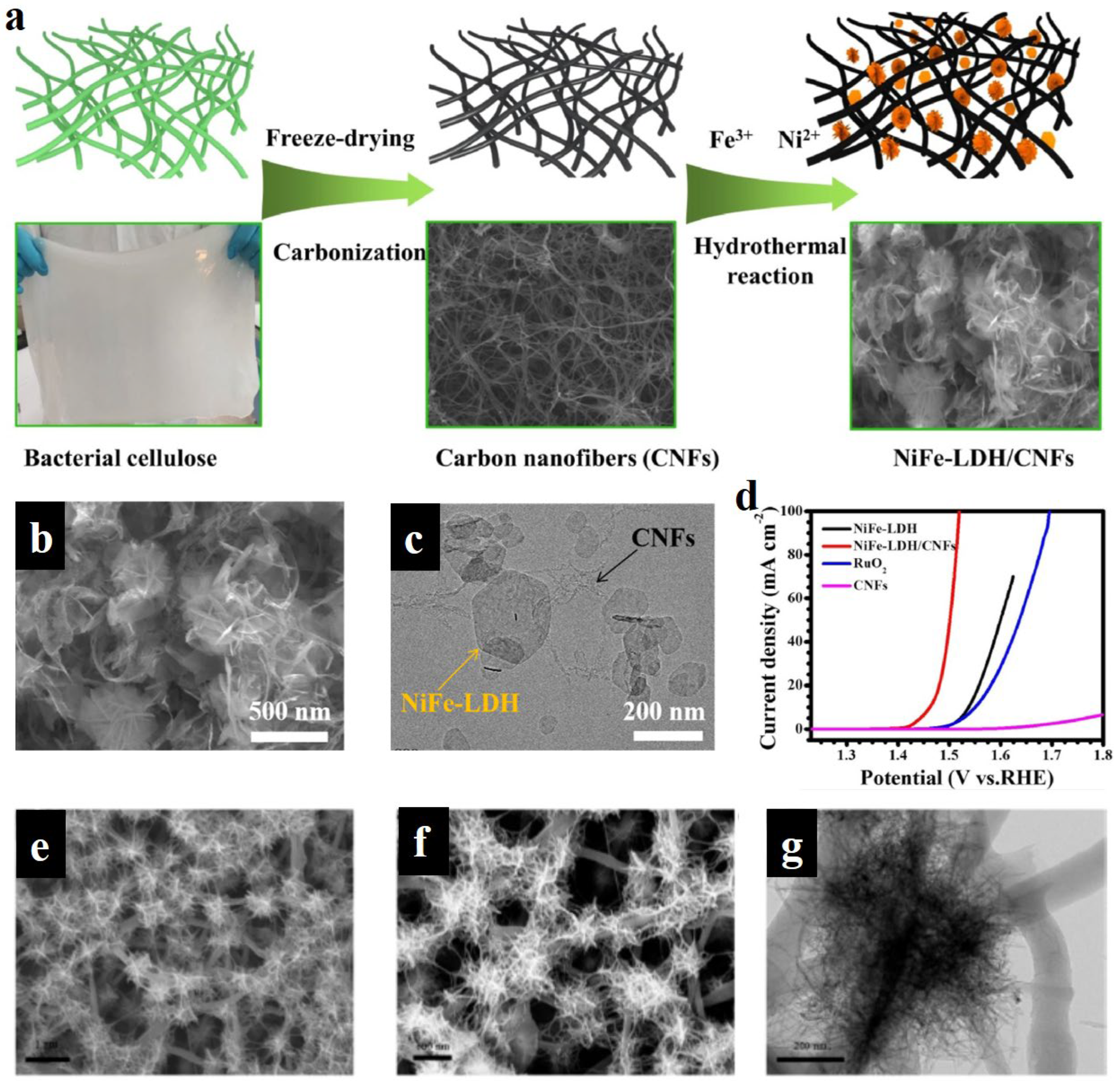
- Shi, Q.; Liu, H.T.; Liang, J.F.; Zhang, Y.H.; Dong, Y.Q.; Yang, W.Y.; Liu, Q. Spatially confined growth of ultrathin NiFe layered double hydroxide nanosheets within carbon nanofibers network for highly efficient water oxidation. Int. J. Hydrogen Energ. 2022, 47, 16047–16055. [Google Scholar] [CrossRef]
- Guo, X.L.; Zheng, X.Q.; Hu, X.L.; Zhao, Q.N.; Li, L.; Yu, P.; Jing, C.; Zhang, Y.X.; Huang, G.S.; Jiang, B.; Xu, C.H.; Pan, F.S. Electrostatic adsorbing graphene quantum dot into nickel-based layered double hydroxides: Electron absorption/donor effects enhanced oxygen electrocatalytic activity. Nano Energy, 2021, 84, 105932. [Google Scholar] [CrossRef]
- Wu, D.; Hu, X.L.; Yang, Z.G.; Yang, T.X.; Wen, J.; Lu, G.J.; Zhao, Q.N.; Li, Z.Y.; Jiang, X.P.; Xu, C.H. NiFe LDH anchoring on Fe/N-doped carbon nanofibers as a bifunctional electrocatalyst for rechargeable zinc-air batteries. Ind. Eng. Chem. Res. 2022, 61, 7523–7528. [Google Scholar] [CrossRef]
- Yin, P.Q.; Wu, G.; Wang, X.Q.; Liu, S.J.; Zhou, F.Y.; Dai, L.; Wang, X.; Yang, B.; Yu, Z.Q. NiCo-LDH nanosheets strongly coupled with GO-CNTs as a hybrid electrocatalyst for oxygen evolution reaction. Nano Research, 2021, 14, 4783–4788. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Wang, X.; Huang, J.F.; Dong, P.P.; Ji, J.; Li, J.; Cao, L.Y.; Feng, L.L.; Jin, P.; Wang, C.R. Decorating CoNi layered double hydroxides nanosheet arrays with fullerene quantum dot anchored on Ni foam for efficient electrocatalytic water splitting and urea electrolysis. Chem Eng J, 2020, 390, 124525. [Google Scholar] [CrossRef]
- Pan, F.H.K.; Huang, K.; Liu, P.F.; Li, R.; Lian, C.; Liu, H.L.; Hu, J. In situ electroactivated Fe-NiOOH nanoclusters on carbon quantum dots for efficient large-scale oxygen production. Small Struct. 2022, 3, 2200094. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, L.C.; Yue, L.C.; Deng, B.; Cao, Y.; Liu, Q.; Luo, Y.L.; Lu, S.Y.; Zheng, B.Z.; Sun, X.P. A NiCo LDH nanosheet array on graphite felt: an efficient 3D electrocatalyst for the oxygen evolution reaction in alkaline media. Inorg. Chem. Front. 2021, 8, 3162–3166. [Google Scholar] [CrossRef]
- Li, F.Y.; Du, M.; Xiao, X.; Xu, Q. Self-supporting metal-organic framework-based nanoarrays for electrocatalysis. ACS Nano 2022. [Google Scholar] [CrossRef]
- Tang, L.; Cai, M.J.; Zhang, M.S.; Chen, X.; Cai, Z.X. LDH-assisted growth of FeCo bimetal-MOF nanorods for electrocatalytic oxygen evolution. RSC Adv. 2022, 12, 25112–25117. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, H.J.; Huang, Y. Electron-rich NiFe layered double hydroxides via interface engineering for boosting electrocatalytic oxygen evolution. Appl. Catal. B-Environ. 2021, 297, 120453. [Google Scholar] [CrossRef]
- Zhang, L.C.; Wang, J.Q.; Liu, P.Y.; Liang, J.; Luo, Y.S.; Cui, G.W.; Tang, B.; Liu, Q.; Yan, X.D.; Hao, H.G.; Liu, M.L.; Gao, R.; Sun, X.P. Ni(OH)2 nanoparticles encapsulated in conductive nanowire array for high-performance alkaline seawater oxidation. Nano Res. 2022, 15, 6084–6090. [Google Scholar] [CrossRef]
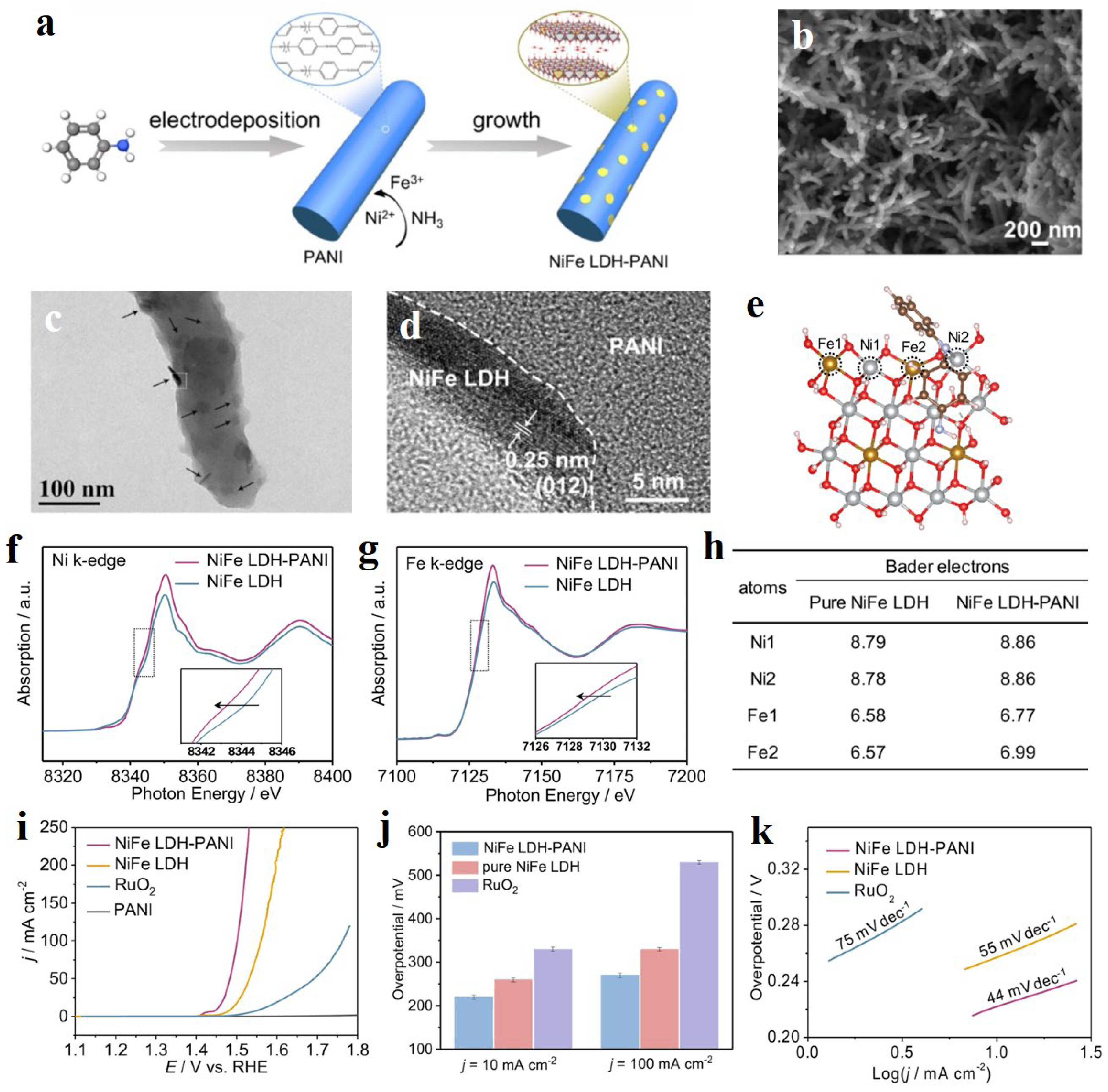
- Zheng, F.Z.; Zhang, W.F.; Zhang, X.X.; Zhang, Y.L.; Chen, W. Sub-2 nm Ultrathin and Robust 2D FeNi Layered Double Hydroxide Nanosheets Packed with 1D FeNi-MOFs for Enhanced Oxygen Evolution Electrocatalysis. Adv. Funct. Mater. 2021, 31, 2103318. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Gao, J.K.; Zhang, Q.C. Current progress in 2D metal-organic frameworks for electrocatalysis. Small Struc. 2022, 3, 2200109. [Google Scholar] [CrossRef]
- Xu, H.W.; Zhang, W.D.; Liu, J.Y.; Yao, Y.; Yan, X.D.; Gu, Z.G. Intercalation-induced partial exfoliation of NiFe LDHs with abundant active edge sites for highly enhanced oxygen evolution reaction. J. Colloid Interf. Sci. 2022, 607, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.S.; Liu, S.; Zhang, S.J.; Cao, Y.; Wang, H.L.; Han, C.Y.; Sun, J. High corrosion Resistance of NiFe-layered double hydroxide catalyst for stable seawater electrolysis promoted by phosphate intercalation. Small 2022, 18, 2203852. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, B.J.; Gaikwad, A.P.; Rode, C.V.; Sathaye, S.D.; Patil, K.R.; Malkhede, D.D. Calixarene intercalated NiCo layered double hydroxide for enhanced oxygen evolution catalysis. ACS Sustainable Chem. Eng. 2018, 6, 9649–9660. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Zhang, C.C.; Xu, Y.; Zhang, W.; Zheng, H.Q.; Cao, R. Dual tuning of ultrathin α-Co(OH)2 nanosheets by solvent engineering and coordination competition for efficient oxygen evolution. ACS Sustainable Chem. Eng. 2019, 7, 3527–3535. [Google Scholar] [CrossRef]
- Laipan, M.W.; Yu, J.F.; Zhu, R.L.; Zhu, J.X.; Smith, A.T.; He, H.P.; O’Hare, D.; Sun, L.Y. Functionalized layered double hydroxides for innovative applications. Mater. Hor. 2020, 7, 715–745. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.L.; Li, D.; Gao, Q.S. Single-layer CoFe hydroxides for efficient electrocatalytic oxygen evolution. Chem. Commun. 2021, 57, 7653–7656. [Google Scholar] [CrossRef]
- Zhang, L.C.; Liang, J.; Yue, L.C.; Dong, K.; Li, J.; Zhao, D.L.; Li, Z.R.; Sun, S.J.; Luo, Y.S.; Liu, Q.; Cui, G.W.; Alshehri, A.A.; Guo, X.D.; Sun, X.P. Benzoate anions-intercalated NiFe-layered double hydroxide nanosheet array with enhanced stability for electrochemical seawater oxidation. Nano Res. Energy 2022, 1, e9120028. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.L.; Yang, L.C.; Gao., Q.S. Recent advances of two-dimensional CoFe layered-double-hydroxides for electrocatalytic water oxidation. Chinese Chem. Lett. 2022, 33, 2845–2855. [Google Scholar] [CrossRef]
- Ahmed, Z.; Krishankant; Rai, R.; Kumar, R.; Maruyama, T.; Bera, C.; Bagchi, V. Unraveling a graphene exfoliation technique analogy in the making of ultrathin nickel-iron oxyhydroxides@nickel foam to promote the OER. ACS Appl. Mater. Interfaces 2021, 13, 55281–55291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Xie, C.; Zhang, Z.Y.; Liu, D.D.; Chen, R.; Wang, S.Y. In situ exfoliated, N-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction. Adv. Funct. Mater. 2018, 28, 1703363. [Google Scholar] [CrossRef]
- S, T.; Munonde.; Zheng, H.T. The impact of ultrasonic parameters on the exfoliation of NiFe LDH nanosheets as electrocatalysts for the oxygen evolution reaction in alkaline media. Ultrason. Sonochem. 2021, 76, 105664. [CrossRef] [PubMed]
- Yan, D.F.; Xia, C.F.; Zhang, W.J.; Hu, Q.; He, C.X.; Xia, B.Y.; Wang, S.Y. Cation defect engineering of transition metal electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2022, 12, 2202317. [Google Scholar] [CrossRef]
- Bai, X.; Duan, Z.Y.; Nan, B.; Wang, L.M.; Tang, T.M.; Guan, J.Q. Unveiling the active sites of ultrathin Co-Fe layered double hydroxides for the oxygen evolution reaction. Chinese J. Catal. 2022, 43, 2240–2248. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, Y.Q.; Liu, Z.J.; Xie, C.; Feng, S.; Liu, D.D.; Shao, M.F.; Wang, S.Y. Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Wu, Y.J.; Yang, J.; Tu, T.X.; Li, W.Q.; Zhang, P.F.; Zhou, Y.; Li, J.F.; Li, J.T.; Sun, S.G. Evolution of cationic vacancy defects: a motif for surface restructuration of OER precatalyst. Angew. Chem. Int. Ed. 2021, 60, 26829–26836. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Tao, S.; Lin, H.; Wang, G.P.; Zhao, K.N.; Cai, R.M.; Tao, K.W.; Zhang, C.X.; Sun, M.Z.; Hu, J.; Huang, B.L.; Yang, S.H. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy 2021, 81, 105606. [Google Scholar] [CrossRef]
- Liu, J.J.; Zheng, M.T.; Li, J.T.; Yuan, Y.F.; Li, C.H.; Zhang, S.Q.; Yang, L.; Bai, Z.Y.; Lu, J. Lithiation-induced defect engineering to promote oxygen evolution reaction. Adv. Funct. Mater. 2023, 33, 2209753. [Google Scholar] [CrossRef]
- Zhai, Y.Y.; Ren, X.R.; Sun, Y.; Li, D.; Wang, B.L.; Liu, S.Z. Synergistic effect of multiple vacancies to induce lattice oxygen redox in NiFe-layered double hydroxide OER catalysts. Appl. Catal. B-Environ. 2023, 323, 122091. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




