Submitted:
17 January 2023
Posted:
25 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
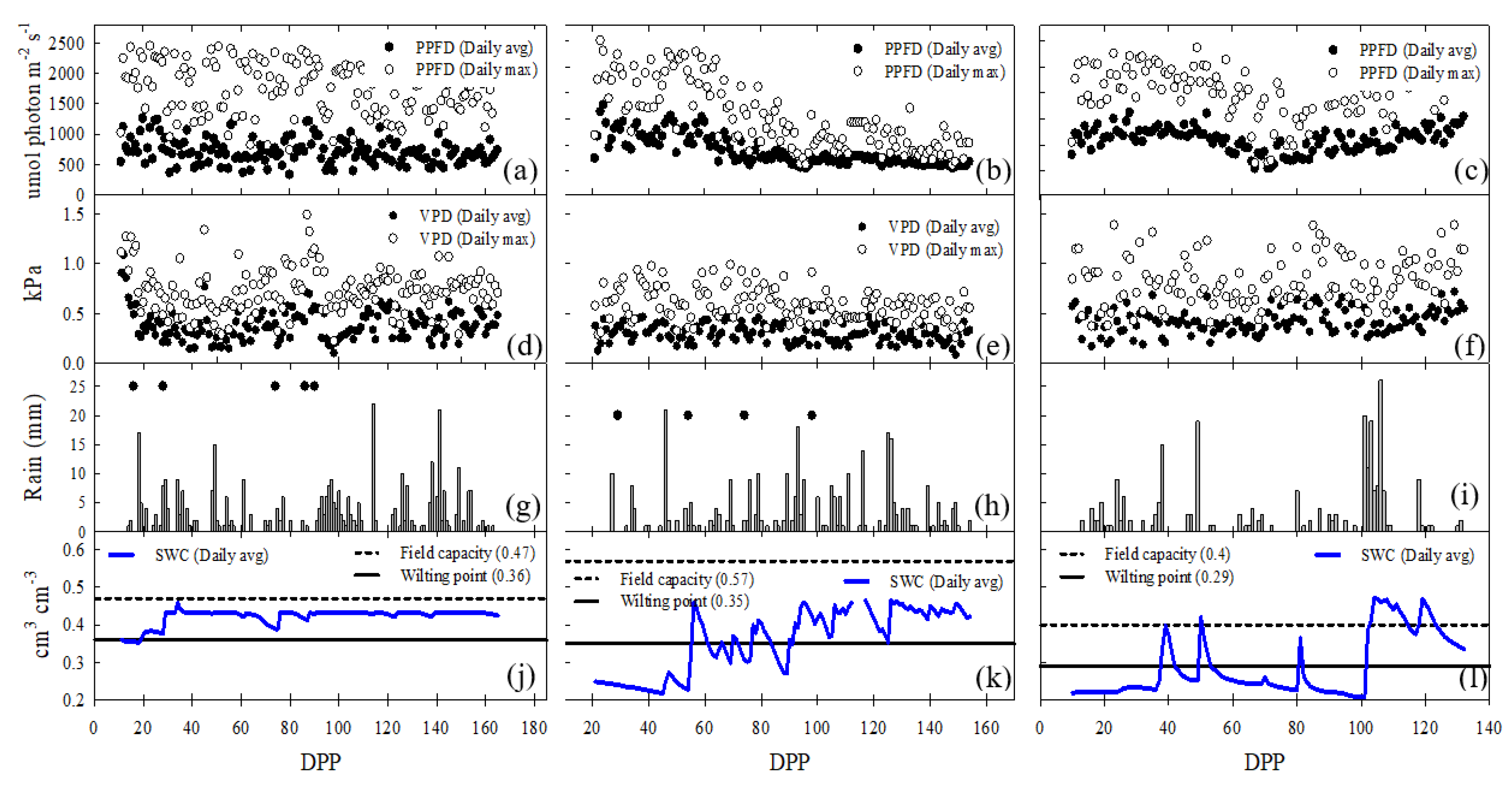
2.1. Site Description
2.2. Meteorological and Eddy Covariance Measurements
2.3. NEE Partitioning between Gross Primary Productivity GPP and Ecosystem Respiration Reco
2.4. ET-GPP Coupling Analysis
2.4.1. Inherent Water Use Efficiency
2.4.2. Diurnal and Daily ET-GPP Coupling and Synchrony
2.4.3. Coupling between the Plant Canopy and the Atmosphere
2.5. Biological Measurements and Growth Analysis
3. Results
3.1. Meteorological Conditions
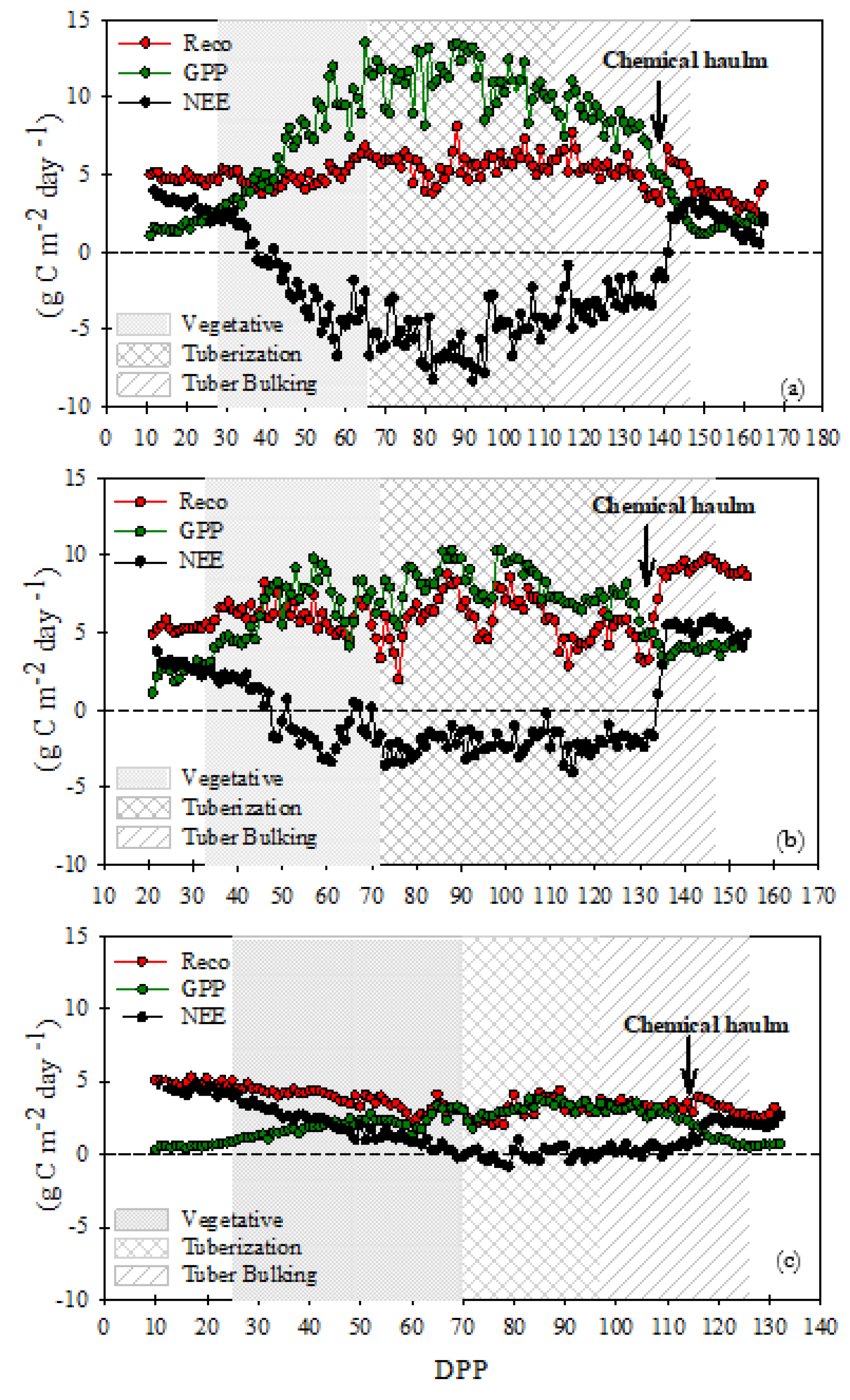
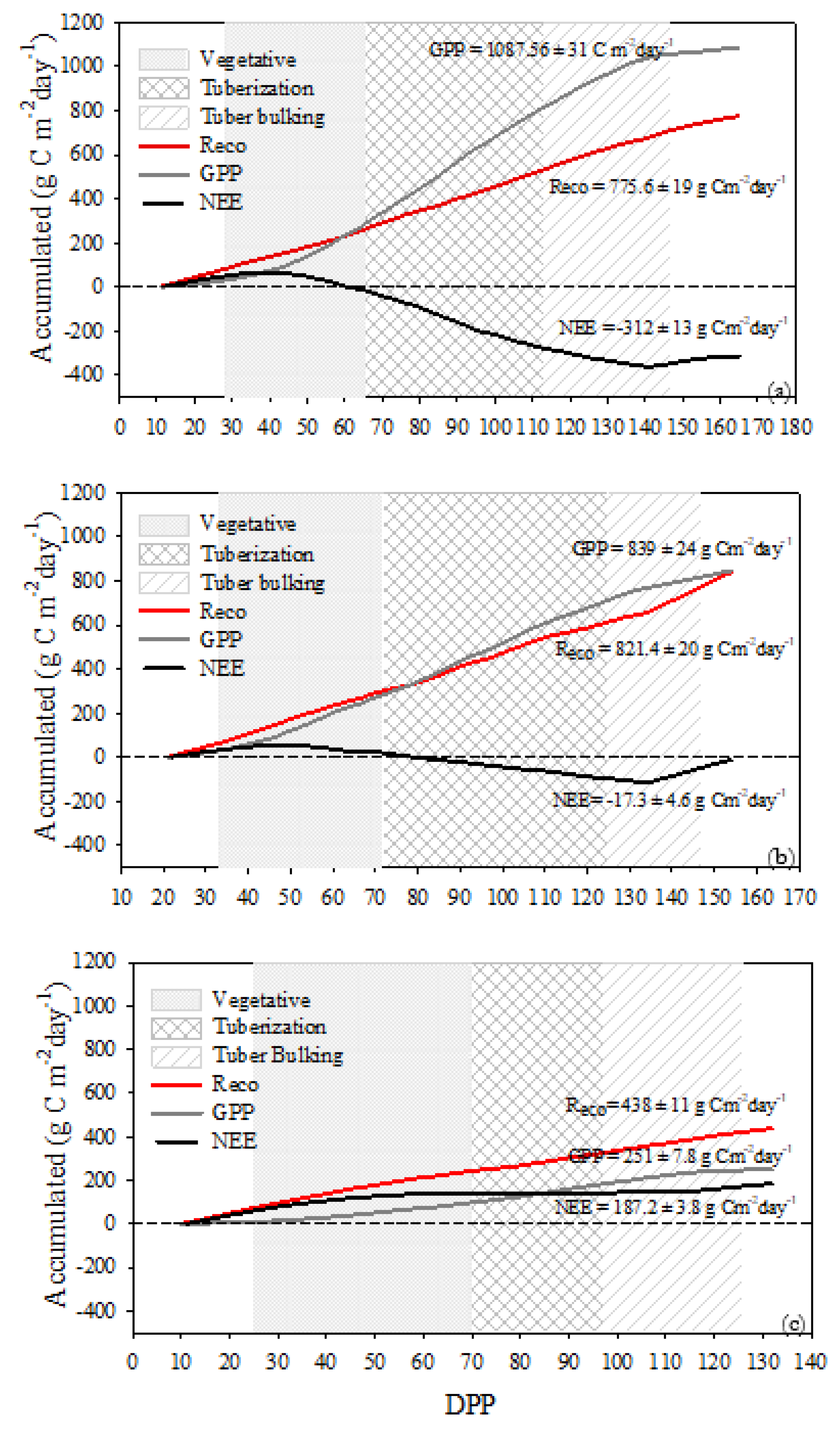
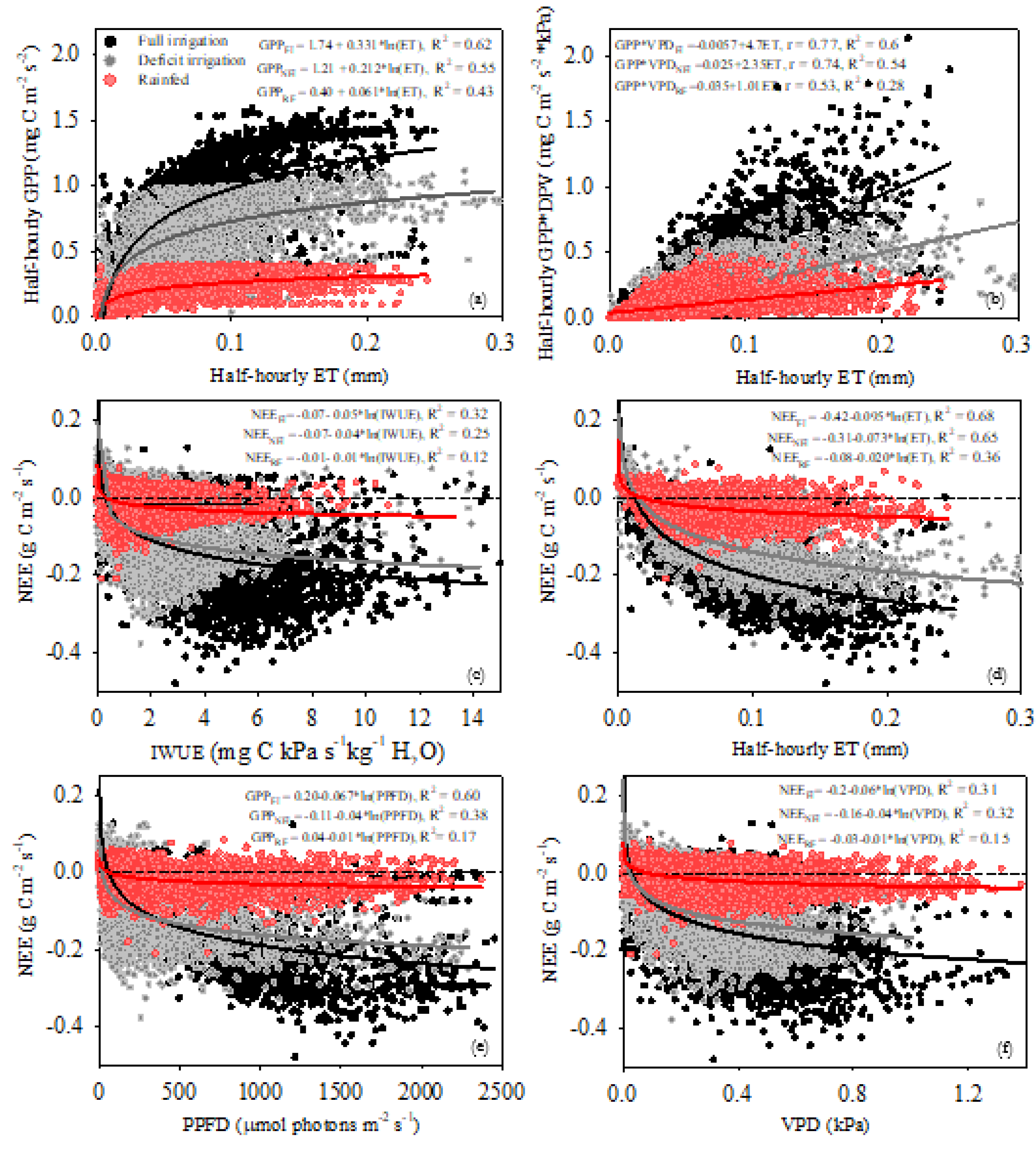
3.2. Carbon Fluxes of NEE, GPP, and Reco
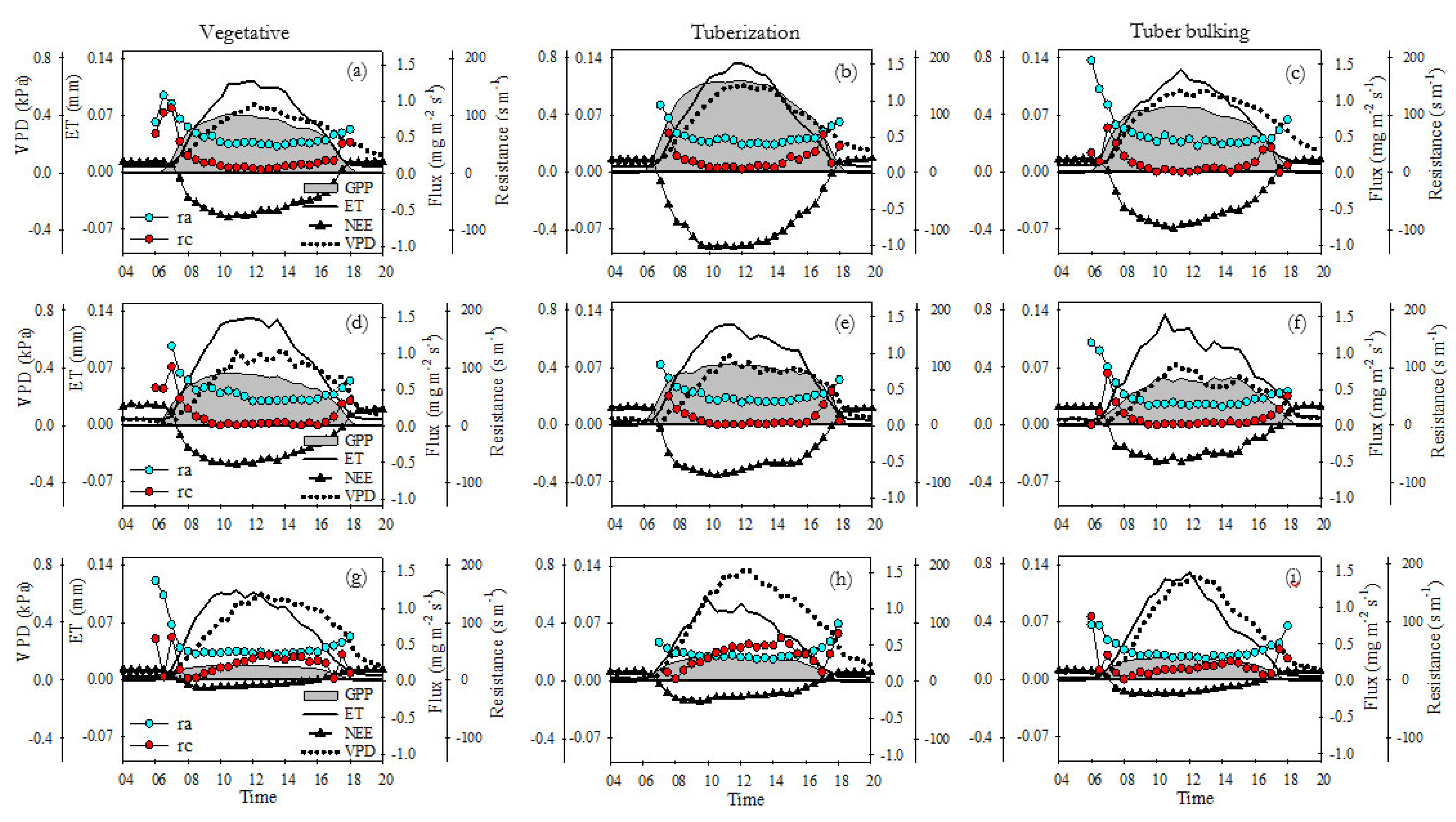
3.3. Crop Development, Surface Resistance and Carbon - Water Fluxes
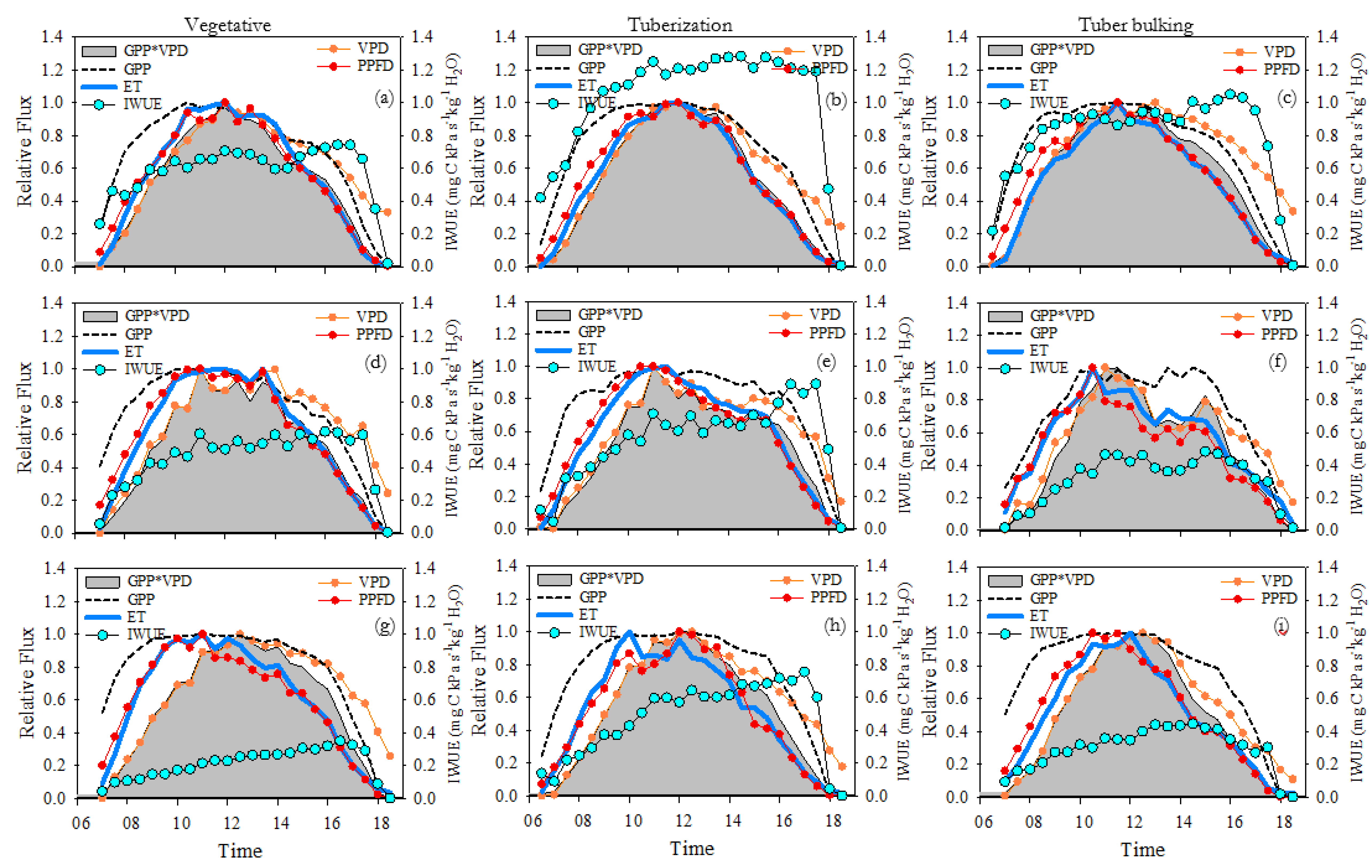
3.4. Diurnal ET-GPP Trends, Synchrony and IWUE
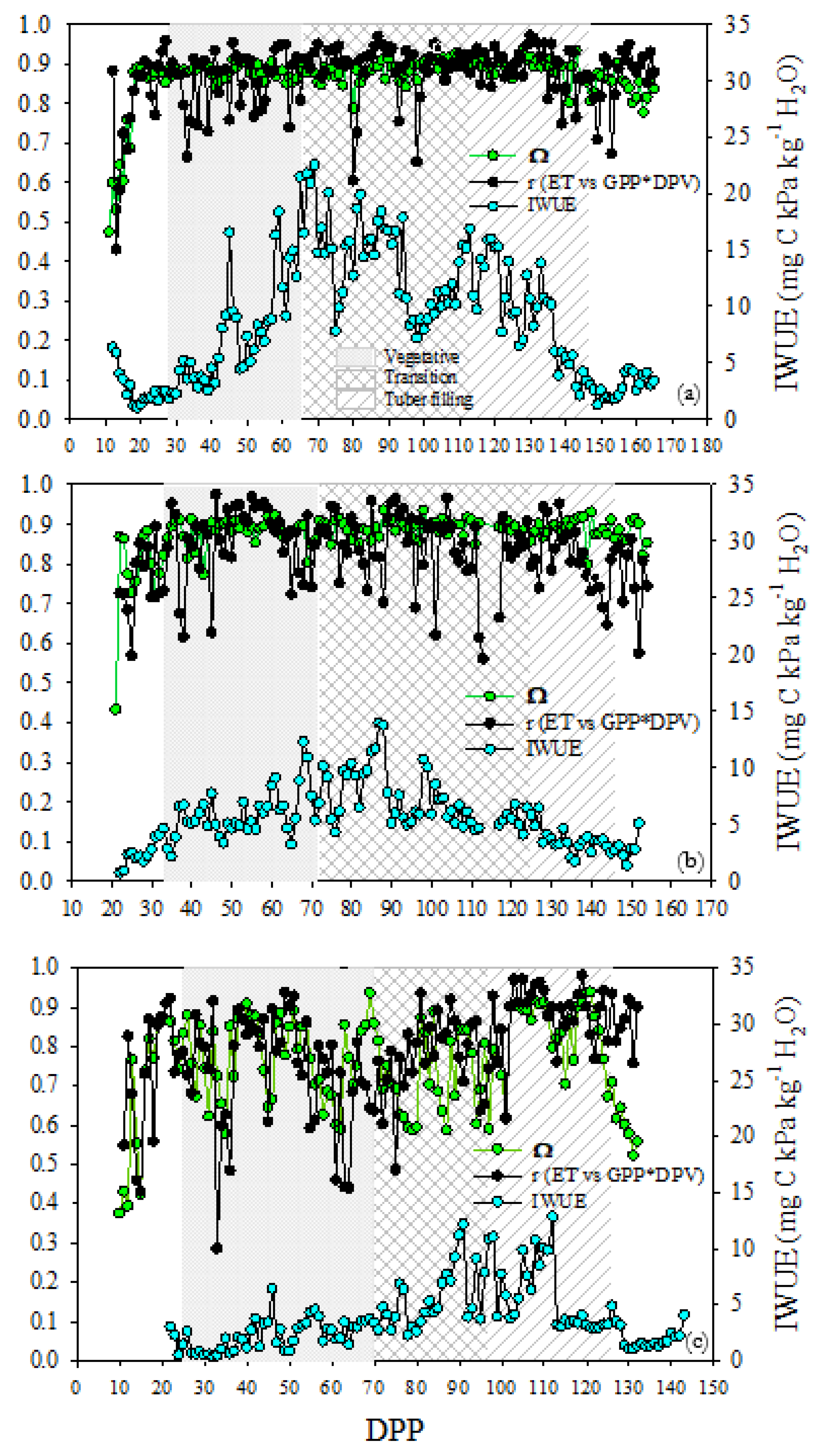
3.5. ET-GPP Coupling and Omega
4. Discussion
4.1. Carbon Fluxes of NEE, GPP, and Reco
4.2. Crop Development, Surface Resistance and Carbon - Water Fluxes
4.3. Environmental Controls on ET-GPP Synchrony and NEE-IWUE Relations
4.4. ET-GPP Coupling and the Omega Role
5. Conclusions
References
- Abbate, P.E.; Dardanelli, J.L., Cantarero. Climatic and Water Availability Effects on Water-Use Efficiency in Wheat. n.d.
- Aguilos, M.; Sun, G.; Noormets, A.; Domec, J.-C.; McNulty, S.; Gavazzi, M.; Prajapati, P.; Minick, K.J.; Mitra, B.; King, J. Ecosystem Productivity and Evapotranspiration Are Tightly Coupled in Loblolly Pine (Pinus taeda L.) Plantations along the Coastal Plain of the Southeastern U.S. Forests 2021, 12, 1123. [Google Scholar] [CrossRef]
- Aires, L.; Pio, C.; Pereira, J. The effect of drought on energy and water vapour exchange above a mediterranean C3/C4 grassland in Southern Portugal. Agric. For. Meteorol. 2008, 148, 565–579. [Google Scholar] [CrossRef]
- Ali, S.; Xu, Y.; Ma, X.; Ahmad, I.; Kamran, M.; Dong, Z.; Cai, T.; Jia, Q.; Ren, X.; Zhang, P.; et al. Planting Patterns and Deficit Irrigation Strategies to Improve Wheat Production and Water Use Efficiency under Simulated Rainfall Conditions. Front. Plant Sci. 2017, 8, 1408. [Google Scholar] [CrossRef]
- Alves, I.; Perrier, A.; Pereira, L.S. AERODYNAMIC AND SURFACE RESISTANCES OF COMPLETE COVER CROPS: HOW GOOD IS THE “BIG LEAF”? Trans. ASAE 1998, 41, 345–351. [Google Scholar] [CrossRef]
- Alves, J.D.N.; Ribeiro, A.; Rody, Y.P.; Loos, R.A. Energy balance and surface decoupling factor of a pasture in the Brazilian Cerrado. Agric. For. Meteorol. 2022, 319, 108912. [Google Scholar] [CrossRef]
- Alves, J.D.N.; Ribeiro, A.; Rody, Y.P.; Loos, R.A. Energy balance and surface decoupling factor of a pasture in the Brazilian Cerrado. Agric. For. Meteorol. 2022, 319, 108912. [Google Scholar] [CrossRef]
- Amer, K.H.; Hatfield, J.L. Canopy Resistance as Affected by Soil and Meteorological Factors in Potato. Agron. J. 2004, 96, 978–985. [Google Scholar] [CrossRef]
- Arkebauer, T.J.; Walter-Shea, E.A.; Mesarch, M.A.; Suyker, A.E.; Verma, S.B. Scaling up of CO2 fluxes from leaf to canopy in maize-based agroecosystems. Agric. For. Meteorol. 2009, 149, 2110–2119. [Google Scholar] [CrossRef]
- Aubinet, M.; Moureaux, C.; Bodson, B.; Dufranne, D.; Heinesch, B.; Suleau, M.; Vancutsem, F.; Vilret, A. Carbon sequestration by a crop over a 4-year sugar beet/winter wheat/seed potato/winter wheat rotation cycle. Agric. For. Meteorol. 2009, 149, 407–418. [Google Scholar] [CrossRef]
- Baldocchi, D.; Kelliher, F.M.; Black, T.A.; Jarvis, P. Climate and vegetation controls on boreal zone energy exchange. Glob. Chang. Biol. 2000, 6, 69–83. [Google Scholar] [CrossRef]
- Battipaglia, G.; Saurer, M.; Cherubini, P.; Calfapietra, C.; McCarthy, H.R.; Norby, R.J.; Cotrufo, M.F. Elevated CO2 increases tree-level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytol. 2012, 197, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Battipaglia, G.; Saurer, M.; Cherubini, P.; Calfapietra, C.; McCarthy, H.R.; Norby, R.J.; Cotrufo, M.F. Elevated CO2 increases tree-level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytol. 2012, 197, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Ciais, P.; Reichstein, M.; Baldocchi, D.; Law, B.E.; Papale, D.; Soussana, J.; Ammann, C.; Buchmann, N.; Frank, D.; et al. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Bierhuizen, J.F.; Slatyer, R.O. Agricultural Meteorology-Elsevier Publishing Company, Amsterdam-Printed in The Netherlands EFFECT OF ATMOSPHERIC CONCENTRATION OF WATER VAPOUR AND COs IN DETERMINING TRANSPIRATION-PHOTOSYNTHESIS RELATIONSHIPS OF COTTON LEAVES, Agr. Meteorol. 1965. [Google Scholar]
- Birch, P.R.J.; Bryan, G.J.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Bouzalakos, S.; Mercedes, M. Overview of carbon dioxide (CO2) capture and storage technology. Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology 1–24. 2010. [Google Scholar] [CrossRef]
- Brown, K.W. Sugar beet and potatoes. In Vegetation and the Atmosphere; Monteith, J.L., Ed.; Academic Press: London, 1976; pp. 65–86. [Google Scholar]
- Brunsell, N.A.; Wilson, C.J. Multiscale Interactions betweenWater and Carbon Fluxes and Environmental Variables in A Central U.S. Grassland. Entropy 2013, 15, 1324–1341. [Google Scholar] [CrossRef]
- Buysse, P.; Bodson, B.; Debacq, A.; De Ligne, A.; Heinesch, B.; Manise, T.; Moureaux, C.; Aubinet, M. Carbon budget measurement over 12 years at a crop production site in the silty-loam region in Belgium. Agric. For. Meteorol. 2017, 246, 241–255. [Google Scholar] [CrossRef]
- Cabral, O.M.; Rocha, H.R.; Gash, J.H.; Ligo, M.A.; Ramos, N.P.; Packer, A.P.; Batista, E.R. Fluxes of CO2 above a sugarcane plantation in Brazil. Agric. For. Meteorol. 2013, 182-183, 54–66. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics, second ed.; Springer-Verlag: New York, 1998. [Google Scholar]
- Chen, S.; Chen, J.; Lin, G.; Zhang, W.; Miao, H.; Wei, L.; Huang, J.; Han, X. Energy balance and partition in Inner Mongolia steppe ecosystems with different land use types. Agric. For. Meteorol. 2009, 149, 1800–1809. [Google Scholar] [CrossRef]
- Chi, J.; Waldo, S.; Pressley, S.; O’keeffe, P.; Huggins, D.; Stöckle, C.; Pan, W.L.; Brooks, E.; Lamb, B. Assessing carbon and water dynamics of no-till and conventional tillage cropping systems in the inland Pacific Northwest US using the eddy covariance method. Agric. For. Meteorol. 2016, 218-219, 37–49. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- CIP, 2022. Hechos y Cifras sobre la Papa [WWW Document]. URL. Available online: www.cipotato.
- Clune, S.; Crossin, E.; Verghese, K. Systematic review of greenhouse gas emissions for different fresh food categories. J. Clean. Prod. 2017, 140, 766–783. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Knauer, J.; Williams, C.A. Ideas and perspectives: how coupled is the vegetation to the boundary layer? Biogeosciences 2017, 14, 4435–4453. [Google Scholar] [CrossRef]
- de la Motte, L.G.; Beauclaire, Q.; Heinesch, B.; Cuntz, M.; Foltýnová, L.; Šigut, L.; Kowalska, N.; Manca, G.; Ballarin, I.G.; Vincke, C.; et al. Non-stomatal processes reduce gross primary productivity in temperate forest ecosystems during severe edaphic drought. Philos. Trans. R. Soc. B: Biol. Sci. 2020, 375, 20190527. [Google Scholar] [CrossRef]
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for Sustainable Global Food Security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Díaz, E.; Adsuara, J.E.; Martínez, M.; Piles, M.; Camps-Valls, G. Inferring causal relations from observational long-term carbon and water fluxes records. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Eamus, D.; Huete, A.; Yu, Q. Modelling Leaf and Canopy Photosynthesis. In Vegetation Dynamics: A Synthesis of Plant Ecophysiology, Remote Sensing and Modelling; Cambridge University Press.: Cambridge, 2016a; pp. 260–280. [Google Scholar] [CrossRef]
- Eamus, D.; Huete, A.; Yu, Q. Modelling Radiation Exchange and Energy Balances of Leaves and Canopies, in: Vegetation Dynamics: A Synthesis of Plant Ecophysiology, Remote Sensing and Modelling. Cambridge University Press., Cambridge, pp. 244–259. In Vegetation Dynamics: A Synthesis of Plant Ecophysiology, Remote Sensing and Modelling; Cambridge University Press.: Cambridge, 2016b; pp. 244–259. [Google Scholar] [CrossRef]
- Evans, J.R. Mesophyll conductance: walls, membranes and spatial complexity. New Phytol. 2020, 229, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Kjelgaard, J.F.; Stockle, C.O. EVALUATI NG SURFACE RESISTANCE FOR ESTIMATING CORN AND POTATO EVAPOTRANSPIRATION WITH THE PENMAN–MONTEITH MODEL. Trans. ASAE 2001, 44. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Tenhunen, J.; Aubinet, M.; Bakwin, P.; Berbigier, P.; Bernhofer, C.; Burba, G.; Clement, R.; Davis, K.J.; et al. Seasonality of ecosystem respiration and gross primary production as derived from FLUXNET measurements. Agric. For. Meteorol. 2002, 113, 53–74. [Google Scholar] [CrossRef]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: from carbon source to sink-driven vegetation modeling. New Phytol. 2013, 201, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Jin, Y.; Zhang, Y.; Sha, L.; Liu, Y.; Song, Q.; Zhou, W.; Liang, N.; Yu, G.; Zhang, L.; et al. Eddy covariance and biometric measurements show that a savanna ecosystem in Southwest China is a carbon sink. Sci. Rep. 2017, 7, srep41025. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.I. Stress Coefficients for Soil Water Balance Combined with Water Stress Indicators for Irrigation Scheduling of Woody Crops. Horticulturae 2017, 3, 38. [Google Scholar] [CrossRef]
- Field, C.B.; Jackson, R.B.; Mooney, H.A. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant, Cell Environ. 1995, 18, 1214–1225. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193-194, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Niinemets; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M.; Rodriguez, P.L.; et al. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Gentilesca, T.; Battipaglia, G.; Borghetti, M.; Colangelo, M.; Altieri, S.; Ferrara, A.M.S.; Lapolla, A.; Rita, A.; Ripullone, F. Evaluating growth and intrinsic water-use efficiency in hardwood and conifer mixed plantations. Trees 2021, 35, 1329–1340. [Google Scholar] [CrossRef]
- Gentine, P.; Green, J.K.; Guérin, M.; Humphrey, V.; I Seneviratne, S.; Zhang, Y.; Zhou, S. Coupling between the terrestrial carbon and water cycles—a review. Environ. Res. Lett. 2019, 14, 083003. [Google Scholar] [CrossRef]
- George, T.S.; Taylor, M.A.; Dodd, I.C.; White, P.J. Climate Change and Consequences for Potato Production: a Review of Tolerance to Emerging Abiotic Stress. Potato Res. 2017, 60, 239–268. [Google Scholar] [CrossRef]
- Gervais, T.; Creelman, A.; Li, X.-Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato Response to Drought Stress Physiological and Growth Basis. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Gervais, T.; Creelman, A.; Li, X.-Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato Response to Drought Stress Physiological and Growth Basis. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ghislain, M.; Núñez, J.; Herrera, M.d.R.; Spooner, D.M. The single Andigenum origin of Neo-Tuberosum potato materials is not supported by microsatellite and plastid marker analyses. Theor. Appl. Genet. 2009, 118, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Gondim, P.S.D.S.; Lima, J.R.D.S.; Antonino, A.C.D.; Hammecker, C.; Da Silva, R.A.B.; Gomes, C.A. Environmental control on water vapour and energy exchanges over grasslands in semiarid region of Brazil. Revista Brasileira de Engenharia Agricola e Ambiental, 19, 3–8. [CrossRef]
- Gonzalez-Paleo, L.; Ravetta, D. Relationship between photosynthetic rate, water use and leaf structure in desert annual and perennial forbs differing in their growth. Photosynthetica 2018, 56, 1177–1187. [Google Scholar] [CrossRef]
- Goorman, R.; Bartual, A.; Paula, S.; Ojeda, F. Enhancement of photosynthesis in post-disturbance resprouts of two co-occurring Mediterranean Erica species. Plant Ecol. 2011, 212, 2023–2033. [Google Scholar] [CrossRef]
- Goulden, M.L.; Wofsy, S.C.; Harden, J.W.; Trumbore, S.E.; Crill, P.M.; Gower, S.T.; Fries, T.; Daube, B.C.; Fan, S.-M.; Sutton, D.J.; et al. Sensitivity of Boreal Forest Carbon Balance to Soil Thaw. Science 1998, 279, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Gessler, A.; Granier, A.; Pollastrini, M.; Bussotti, F.; Bonal, D. Interspecific competition influences the response of oak transpiration to increasing drought stress in a mixed Mediterranean forest. For. Ecol. Manag. 2014, 318, 54–61. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, B.; Chen, J.; Yan, Y.; Li, B.; Chen, J. Seasonal changes of energy fluxes in an estuarine wetland of Shanghai, China. Chin. Geogr. Sci. 2010, 20, 23–29. [Google Scholar] [CrossRef]
- Guo, R.; Zhao, Y.; Shi, Y.; Li, F.; Hu, J.; Yang, H. Low carbon development and local sustainability from a carbon balance perspective. Resour. Conserv. Recycl. 2017, 122, 270–279. [Google Scholar] [CrossRef]
- Haile-Mariam, S.; Collins, H.P.; Higgins, S.S. Greenhouse Gas Fluxes from an Irrigated Sweet Corn ( Zea mays L.)–Potato ( Solanum tuberosum L.) Rotation. J. Environ. Qual. 2008, 37, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Herbst, M.; Kutsch, W.L.; Hummelshøj, P.; Jensen, N.O.; Kappen, L. Canopy physiology: interpreting the variations in eddy fluxes of water vapour and carbon dioxide observed over a beech forest Basic and Applied Ecology. Basic Appl. Ecol 2002. [Google Scholar] [CrossRef]
- Hill, D.; Nelson, D.; Hammond, J.; Bell, L. Morphophysiology of Potato (Solanum tuberosum) in Response to Drought Stress: Paving the Way Forward. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Nelson, D.; Hammond, J.; Bell, L. Morphophysiology of Potato (Solanum tuberosum) in Response to Drought Stress: Paving the Way Forward. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Howlader, O.; Hoque, M. Growth analysis and yield performance of four potato (Solanum tuberosum L.) Varieties. Bangladesh J. Agric. Res. 2018, 43, 267–280. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, G.; Fu, Y.; Sun, X.; Li, Y.; Shi, P.; Wang, Y.; Zheng, Z. Effects of vegetation control on ecosystem water use efficiency within and among four grassland ecosystems in China. Glob. Chang. Biol. 2008, 14, 1609–1619. [Google Scholar] [CrossRef]
- Hunt, R. Basic Growth Analysis; Springer Science and Business Media LLC: Dordrecht, GX, Netherlands, 1990. [Google Scholar]
- Huxman, T.E.; Snyder, K.A.; Tissue, D.; Leffler, A.J.; Ogle, K.; Pockman, W.T.; Sandquist, D.R.; Potts, D.L.; Schwinning, S. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 2004, 141, 254–268. [Google Scholar] [CrossRef]
- Irmak, S.; Mutiibwa, D. On the dynamics of canopy resistance: Generalized linear estimation and relationships with primary micrometeorological variables. Water Resour. Res. 2010, 46. [Google Scholar] [CrossRef]
- Jarvis, P.P. COUPLING OF TRANSPIRATION TO THE ATMOSPHERE IN HORTICULTURAL CROPS: THE OMEGA FACTOR. Acta Hortic. 1985, 187–206. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal Control of Transpiration: Scaling Up from Leaf to Region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal Control of Transpiration: Scaling Up from Leaf to Region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar] [CrossRef]
- Jennings, S.A.; Koehler, A.-K.; Nicklin, K.J.; Deva, C.; Sait, S.M.; Challinor, A.J. Global Potato Yields Increase Under Climate Change With Adaptation and CO2 Fertilisation. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.S.; Wu, B.; Zhang, Y.Q.; Gong, J.N.; Qin, S.G.; Chen, G.P.; Qian, D.; Kellomäki, S.; Peltola, H. Biophysical controls on net ecosystem CO2 exchange over a semiarid shrubland in northwest China. Biogeosciences 2014, 11, 4679–4693. [Google Scholar] [CrossRef]
- Jongen, M.; Pereira, J.S.; Aires, L.M.I.; Pio, C.A. The effects of drought and timing of precipitation on the inter-annual variation in ecosystem-atmosphere exchange in a Mediterranean grassland. Agric. For. Meteorol. 2011, 151, 595–606. [Google Scholar] [CrossRef]
- Jullien, A.; Allirand, J.-M.; Mathieu, A.; Andrieu, B.; Ney, B. Variations in leaf mass per area according to N nutrition, plant age, and leaf position reflect ontogenetic plasticity in winter oilseed rape (Brassica napus L.). Field Crop. Res. 2009, 114, 188–197. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Z.; Noormets, A.; Fang, X.; Zha, T.; Zhou, J.; Sun, G.; McNulty, S.G.; Chen, J. Energy partitioning and surface resistance of a poplar plantation in northern China. Biogeosciences 2015, 12, 4245–4259. [Google Scholar] [CrossRef]
- Katul, G.; Manzoni, S.; Palmroth, S.; Oren, R. A stomatal optimization theory to describe the effects of atmospheric CO2 on leaf photosynthesis and transpiration. Ann. Bot. 2009, 105, 431–442. [Google Scholar] [CrossRef]
- Katul, G.; Manzoni, S.; Palmroth, S.; Oren, R. A stomatal optimization theory to describe the effects of atmospheric CO2 on leaf photosynthesis and transpiration. Ann. Bot. 2009, 105, 431–442. [Google Scholar] [CrossRef]
- Katul, G.G.; Palmroth, S.; Oren, R. Leaf stomatal responses to vapour pressure deficit under current and CO2-enriched atmosphere explained by the economics of gas exchange. Plant, Cell Environ. 2009, 32, 968–979. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef]
- Krich, C.; Mahecha, M.D.; Migliavacca, M.; De Kauwe, M.G.; Griebel, A.; Runge, J.; Miralles, D.G. Decoupling between ecosystem photosynthesis and transpiration: a last resort against overheating. Environ. Res. Lett. 2022, 17, 044013. [Google Scholar] [CrossRef]
- Kumagai, T.; Tateishi, M.; Shimizu, T.; Otsuki, K. Transpiration and canopy conductance at two slope positions in a Japanese cedar forest watershed. Agric. For. Meteorol. 2008, 148, 1444–1455. [Google Scholar] [CrossRef]
- Launiainen, S.; Katul, G.G.; Kolari, P.; Vesala, T.; Hari, P. Empirical and optimal stomatal controls on leaf and ecosystem level CO2 and H2O exchange rates. Agric. For. Meteorol. 2011, 151, 1672–1689. [Google Scholar] [CrossRef]
- Law, B.; Falge, E.; Gu, L.; Baldocchi, D.; Bakwin, P.; Berbigier, P.; Davis, K.; Dolman, A.; Falk, M.; Fuentes, J.; et al. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric. For. Meteorol. 2002, 113, 97–120. [Google Scholar] [CrossRef]
- Law, B.E.; Williams, M.; Anthoni, P.M.; Baldocchi, D.D.; Unsworth, M.H. Measuring and modelling seasonal variation of carbon dioxide and water vapour exchange of a Pinus ponderosa forest subject to soil water deficit. Glob. Chang. Biol. 2000, 6, 613–630. [Google Scholar] [CrossRef]
- Leonardi, S.; Gentilesca, T.; Guerrieri, R.; Ripullone, F.; Magnani, F.; Mencuccini, M.; Noije, T.V.; Borghetti, M. Assessing the effects of nitrogen deposition and climate on carbon isotope discrimination and intrinsic water-use efficiency of angiosperm and conifer trees under rising CO2 conditions. Glob. Chang. Biol. 2012, 18, 2925–2944. [Google Scholar] [CrossRef]
- Leuning, R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant, Cell Environ. 1995, 18, 339–355. [Google Scholar] [CrossRef]
- Li, D.; Fang, K.; Li, Y.; Chen, D.; Liu, X.; Dong, Z.; Zhou, F.; Guo, G.; Shi, F.; Xu, C.; et al. Climate, intrinsic water-use efficiency and tree growth over the past 150 years in humid subtropical China. PLOS ONE 2017, 12, e0172045. [Google Scholar] [CrossRef]
- Li, S.; Lai, C.; Lee, G.; Shimoda, S.; Yokoyama, T.; Higuchi, A.; Oikawa, T. Evapotranspiration from a wet temperate grassland and its sensitivity to microenvironmental variables. Hydrol. Process. 2004, 19, 517–532. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Shao, Q.; Fan, J.; Chen, Z.; Dong, J.; Hu, Z.; Zhan, Y. Community Composition and Structure Affect Ecosystem and Canopy Water Use Efficiency Across Three Typical Alpine Ecosystems. Front. Plant Sci. 2022, 12, 771424. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-S.; Lei, H.; Hu, M.-C.; Visessri, S.; Hsieh, C.-I. Canopy Resistance and Estimation of Evapotranspiration above a Humid Cypress Forest. Adv. Meteorol. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Medlyn, B.E.; Duursma, R.A.; Prentice, I.C.; Wang, H.; Baig, S.; Eamus, D.; de Dios, V.R.; Mitchell, P.; Ellsworth, D.S.; et al. Optimal stomatal behaviour around the world. Nat. Clim. Chang. 2015, 5, 459–464. [Google Scholar] [CrossRef]
- Linderson, M.-L.; Mikkelsen, T.; Ibrom, A.; Lindroth, A.; Ro-Poulsen, H.; Pilegaard, K. Up-scaling of water use efficiency from leaf to canopy as based on leaf gas exchange relationships and the modeled in-canopy light distribution. Agric. For. Meteorol. 2012, 152, 201–211. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Liu, S.; Baret, F.; Abichou, M.; Manceau, L.; Andrieu, B.; Weiss, M.; Martre, P. Importance of the description of light interception in crop growth models. Plant Physiol. 2021, 186, 977–997. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Song, H.; Zeng, X. Determinants of net primary productivity: Low-carbon development from the perspective of carbon sequestration. Technol. Forecast. Soc. Chang. 2021, 172, 121006. [Google Scholar] [CrossRef]
- Loader, N.J.; Walsh, R.P.D.; Robertson, I.; Bidin, K.; Ong, R.C.; Reynolds, G.; McCarroll, D.; Gagen, M.; Young, G.H.F. Recent trends in the intrinsic water-use efficiency of ringless rainforest trees in Borneo. Philos. Trans. R. Soc. B: Biol. Sci. 2011, 366, 3330–3339. [Google Scholar] [CrossRef]
- Lombardozzi, D.; Levis, S.; Bonan, G.; Sparks, J.P. Predicting photosynthesis and transpiration responses to ozone: decoupling modeled photosynthesis and stomatal conductance. Biogeosciences 2012, 9, 3113–3130. [Google Scholar] [CrossRef]
- López-Olivari, R.; Fuentes, S.; Poblete-Echeverría, C.; Quintulen-Ancapi, V.; Medina, L. Site-Specific Evaluation of Canopy Resistance Models for Estimating Evapotranspiration over a Drip-Irrigated Potato Crop in Southern Chile under Water-Limited Conditions. Water 2022, 14, 2041. [Google Scholar] [CrossRef]
- Mackay, G.R. New Light on a Hidden Treasure. Rome: FAO (2009), pp. 136, US$45.00. ISBN 978-92-5-106142-8. Exp. Agric. 2009, 45, 376–376. [Google Scholar] [CrossRef]
- Magnani, F.; Leonardi, S.; Tognetti, R.; Grace, J.; Borghetti, M. Modelling the surface conductance of a broad-leaf canopy: effects of partial decoupling from the atmosphere. Plant, Cell Environ. 1998, 21, 867–879. [Google Scholar] [CrossRef]
- Mallick, K.; Trebs, I.; Boegh, E.; Giustarini, L.; Schlerf, M.; Drewry, D.T.; Hoffmann, L.; von Randow, C.; Kruijt, B.; Araùjo, A.; et al. Canopy-scale biophysical controls of transpiration and evaporation in the Amazon Basin. Hydrol. Earth Syst. Sci. 2016, 20, 4237–4264. [Google Scholar] [CrossRef]
- Martínez-Maldonado, F.E.; Castaño-Marín, A.M.; Goez-Vinasco, G.A.; Marin, F.R. Gross Primary Production of Rainfed and Irrigated Potato ( Solanum tuberosum L.) in the Colombian Andean Region Using Eddy Covariance Technique. Water (Switzerland) 2021a, 13. [Google Scholar] [CrossRef]
- Martínez-Maldonado, F.E.; Castaño-Marín, A.M.; Góez-Vinasco, G.A.; Marin, F.R. Gross Primary Production of Rainfed and Irrigated Potato (Solanum tuberosum L.) in the Colombian Andean Region Using Eddy Covariance Technique. Water 2021, 13, 3223. [Google Scholar] [CrossRef]
- McNaughton, K.; Jarvis, P. Effects of spatial scale on stomatal control of transpiration. Agric. For. Meteorol. 1991, 54, 279–302. [Google Scholar] [CrossRef]
- Meshalkina, J.; Yaroslavtsev, A.; Vassenev, I. Carbon balance of the typical grain crop rotation in Moscow region assessed by eddy covariance method. 2017, 19, 12212. [Google Scholar]
- Meshalkina, J.L.; Yaroslavtsev, A.M.; I Vasenev, I.; Andreeva, I.V.; Tihonova, M.V. Carbon balance assessment by eddy covariance method for agroecosystems with potato plants and oats & vetch mixture on sod-podzolic soils of Russia. IOP Conf. Series: Earth Environ. Sci. 2018, 107, 012119. [Google Scholar] [CrossRef]
- Michel, A.J.; Teixeira, E.I.; Brown, H.E.; Dellow, S.J.; Maley, S.; Gillespie, R.N.; Richards, K.K. Water stress responses of three potato cultivars. Agronomy New Zealand 2019, 49, 25–37. [Google Scholar]
- Migliavacca, M.; Meroni, M.; Manca, G.; Matteucci, G.; Montagnani, L.; Grassi, G.; Zenone, T.; Teobaldelli, M.; Goded, I.; Colombo, R.; et al. Seasonal and interannual patterns of carbon and water fluxes of a poplar plantation under peculiar eco-climatic conditions. Agric. For. Meteorol. 2009, 149, 1460–1476. [Google Scholar] [CrossRef]
- Katz, A. The determination of rubidium and strontium in silicates by flameless atomic-absorption spectroscopy. Chem. Geol. 1975, 16, 15–25. [Google Scholar] [CrossRef]
- Monteith, J.L. How Do Crops Manipulate Water Supply and Demand? Philos Trans R Soc Lond 1986, 316, 245–259. [Google Scholar]
- Moors, E.J.; Jacobs, C.; Jans, W.; Supit, I.; Kutsch, W.L.; Bernhofer, C.; Béziat, P.; Buchmann, N.; Carrara, A.; Ceschia, E.; et al. Variability in carbon exchange of European croplands. Agric. Ecosyst. Environ. 2010, 139, 325–335. [Google Scholar] [CrossRef]
- Morén, A.-S.; Lindroth, A.; Grelle, A. Water-use efficiency as a means of modelling net assimilation in boreal forests. Trees 2000, 15, 67–74. [Google Scholar] [CrossRef]
- Vásquez, T.M.; Del Castillo, S.; Gálvez, D.C.; Rodríguez, L.E. Breeding Differently: Participatory Selection and Scaling Up Innovations in Colombia. Potato Res. 2017, 60, 361–381. [Google Scholar] [CrossRef]
- Muthoni, J.; Kabira, J. Potato Production under Drought Conditions: Identification of Adaptive Traits. Int. J. Hortic. 2016, 6. [Google Scholar] [CrossRef]
- Nadal-Sala, D.; Grote, R.; Birami, B.; Knüver, T.; Rehschuh, R.; Schwarz, S.; Ruehr, N.K. Leaf Shedding and Non-Stomatal Limitations of Photosynthesis Mitigate Hydraulic Conductance Losses in Scots Pine Saplings During Severe Drought Stress. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Nassif, D.S.P.; da Costa, L.G.; Vianna, M.d.S.; Carvalho, K.d.S.; Marin, F.R. The role of decoupling factor on sugarcane crop water use under tropical conditions. Exp. Agric. 2019, 55, 913–923. [Google Scholar] [CrossRef]
- Nassif, D.S.P.; Marin, F.R.; Costa, L.G. Evapotranspiration and Transpiration Coupling to the Atmosphere of Sugarcane in Southern Brazil: Scaling Up from Leaf to Field. Sugar Tech 2013, 16, 250–254. [Google Scholar] [CrossRef]
- Nelson, J.A.; Carvalhais, N.; Migliavacca, M.; Reichstein, M.; Jung, M. Water-stress-induced breakdown of carbon–water relations: indicators from diurnal FLUXNET patterns. Biogeosciences 2018, 15, 2433–2447. [Google Scholar] [CrossRef]
- Nelson, J.A.; Carvalhais, N.; Migliavacca, M.; Reichstein, M.; Jung, M. Water-stress-induced breakdown of carbon–water relations: indicators from diurnal FLUXNET patterns. Biogeosciences 2018, 15, 2433–2447. [Google Scholar] [CrossRef]
- Nemecek, T.; Weiler, K.; Plassmann, K.; Schnetzer, J.; Gaillard, G.; Jefferies, D.; García–Suárez, T.; King, H.; i Canals, L.M. Estimation of the variability in global warming potential of worldwide crop production using a modular extrapolation approach. J. Clean. Prod. 2012, 31, 106–117. [Google Scholar] [CrossRef]
- Niinemets; Cescatti, A.; Rodeghiero, M.; Tosens, T. Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant, Cell Environ. 2006, 29, 1159–1178. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xing, X.; Zhang, Z.; Xia, J.; Zhou, X.; Song, B.; Li, L.; Wan, S. Water-use efficiency in response to climate change: from leaf to ecosystem in a temperate steppe. Glob. Chang. Biol. 2011, 17, 1073–1082. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Brown, H.E.; Moot, D.J. Assessing potato canopy growth and development at the individual leaf level to improve the understanding of the plant source–sink relations. New Zealand J. Crop. Hortic. Sci. 2021, 49, 1–22. [Google Scholar] [CrossRef]
- Oo, A.Z.; Yamamoto, A.; Ono, K.; Umamageswari, C.; Mano, M.; Vanitha, K.; Elayakumar, P.; Matsuura, S.; Bama, K.S.; Raju, M.; et al. Ecosystem carbon dioxide exchange and water use efficiency in a triple-cropping rice paddy in Southern India: A two-year field observation. Sci. Total. Environ. 2023, 854, 158541. [Google Scholar] [CrossRef]
- Maroneze, M. M.; Zepka, L. Q.; Vieira, J. G.; Queiroz, M. I.; Jacob-Lopes, E. A Tecnologia de Remoção de Fósforo: Gerenciamento Do Elemento Em Resíduos Industriais. Rev. Ambient. e Agua 2014, 9, 445–458. [Google Scholar] [CrossRef]
- Pereira, J.S.; Mateus, J.A.; Aires, L.M.; Pita, G.; Pio, C.; David, J.S.; Andrade, V.; Banza, J.; David, T.S.; Paço, T.A.; et al. Net ecosystem carbon exchange in three contrasting Mediterranean ecosystems – the effect of drought. Biogeosciences 2007, 4, 791–802. [Google Scholar] [CrossRef]
- Perez, P.J.; Lecina, S.; Castellvi, F.; Martínez-Cob, A.; Villalobos, F.J. A simple parameterization of bulk canopy resistance from climatic variables for estimating hourly evapotranspiration. Hydrol. Process. 2005, 20, 515–532. [Google Scholar] [CrossRef]
- Raker, C.M.; Spooner, D.M. Chilean Tetraploid Cultivated Potato, Solanum tuberosum, is Distinct from the Andean Populations. Crop. Sci. 2002, 42, 1451–1458. [Google Scholar] [CrossRef]
- Rana, G.; Ferrara, R.; Vitale, D.; D’andrea, L.; Palumbo, A. Carbon assimilation and water use efficiency of a perennial bioenergy crop ( Cynara cardunculus L.) in Mediterranean environment. Agric. For. Meteorol. 2016, 217, 137–150. [Google Scholar] [CrossRef]
- Rana, G.; Katerji, N.; Mastrorilli, M.; Moujabber, M.E. Theoretical and Applied Climatology Evapotranspiration and Canopy Resistance of Grass in a Mediterranean Region. Theor. Appl. Climatol. 1994. [Google Scholar] [CrossRef]
- Reichstein, M.; Tenhunen, J.D.; Roupsard, O.; Ourcival, J.-M.; Rambal, S.; Miglietta, F.; Peressotti, A.; Pecchiari, M.; Tirone, G.; Valentini, R. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: Revision of current hypotheses? Glob. Chang. Biol. 2002, 8, 999–1017. [Google Scholar] [CrossRef]
- Rodríguez P., L.; Sanjuanelo C., D.; Ñústez, L.C.E.; Moreno-Fonseca, L.P. Growth and phenology of three Andean potato varieties (Solanum tuberosum L.) under water stress. Agron. Colomb. 2016, 34, 141. [Google Scholar] [CrossRef]
- Samanta, S.; Banerjee, S.; Mukherjee, A.; Patra, P.K.; Chakraborty, P.K. Determining the radiation use efficiency of potato using sunshine hour data: a simple and costless approach. Span. J. Agric. Res. 2020, 18, e0801. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Williams, C.A.; Schaefer, K.; Arneth, A.; Bonal, D.; Buchmann, N.; Chen, J.; Law, B.E.; Lindroth, A.; Luyssaert, S.; et al. Assimilation exceeds respiration sensitivity to drought: A FLUXNET synthesis. Glob. Chang. Biol. 2010, 16, 657–670. [Google Scholar] [CrossRef]
- Scott, R.L.; Huxman, T.E.; Cable, W.L.; Emmerich, W.E. Partitioning of evapotranspiration and its relation to carbon dioxide exchange in a Chihuahuan Desert shrubland. Hydrol. Process. 2006, 20, 3227–3243. [Google Scholar] [CrossRef]
- Scott, R.L.; Huxman, T.E.; Cable, W.L.; Emmerich, W.E. Partitioning of evapotranspiration and its relation to carbon dioxide exchange in a Chihuahuan Desert shrubland. Hydrol. Process. 2006, 20, 3227–3243. [Google Scholar] [CrossRef]
- Service-USDA, U.S.D. of A.N.R.C., 2014. Claves para la Taxonomía de Suelos. Mdp.Edu.Ar 339.
- Shao, J.; Zhou, X.; Luo, Y.; Li, B.; Aurela, M.; Billesbach, D.; Blanken, P.D.; Bracho, R.; Chen, J.; Fischer, M.; et al. Biotic and climatic controls on interannual variability in carbon fluxes across terrestrial ecosystems. Agric. For. Meteorol. 2015, 205, 11–22. [Google Scholar] [CrossRef]
- da Silva, P.F.; Lima, J.R.d.S.; Antonino, A.C.D.; Souza, R.; de Souza, E.S.; Silva, J.R.I.; Alves, E.M. Seasonal patterns of carbon dioxide, water and energy fluxes over the Caatinga and grassland in the semi-arid region of Brazil. J. Arid. Environ. 2017, 147, 71–82. [Google Scholar] [CrossRef]
- Smith, W.K. Importance of Aerodynamic Resistance to Water Use Efficiency in Three Conifers under Field Conditions. Plant Physiol. 1980, 65, 132–135. [Google Scholar] [CrossRef]
- Souza, P.J.d.O.P.d.; Ribeiro, A.; da Rocha, E.J.P.; Farias, J.R.B.; de Souza, E.B. Sazonalidade no balanço de energia em áreas de cultivo de soja na Amazônia. Bragantia 2012, 71, 548–557. [Google Scholar] [CrossRef]
- Spinelli, G.M.; Snyder, R.L.; Sanden, B.L.; Gilbert, M.; Shackel, K.A. Low and variable atmospheric coupling in irrigated Almond (Prunus dulcis) canopies indicates a limited influence of stomata on orchard evapotranspiration. Agric. Water Manag. 2018, 196, 57–65. [Google Scholar] [CrossRef]
- Spinelli, G.M.; Snyder, R.L.; Sanden, B.L.; Gilbert, M.; Shackel, K.A. Low and variable atmospheric coupling in irrigated Almond (Prunus dulcis) canopies indicates a limited influence of stomata on orchard evapotranspiration. Agric. Water Manag. 2018, 196, 57–65. [Google Scholar] [CrossRef]
- Spinelli, G.M.; Snyder, R.L.; Sanden, B.L.; Shackel, K.A. Water stress causes stomatal closure but does not reduce canopy evapotranspiration in almond. Agric. Water Manag. 2016, 168, 11–22. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C. Maize canopies under two soil water regimes: III. Variation in coupling with the atmosphere and the role of leaf area index. Agric. For. Meteorol. 1998, 89, 201–213. [Google Scholar] [CrossRef]
- Sugiura, D.; Terashima, I.; Evans, J.R. A Decrease in Mesophyll Conductance by Cell-Wall Thickening Contributes to Photosynthetic Downregulation1. Plant Physiol. 2020, 183, 1600–1611. [Google Scholar] [CrossRef]
- Sutherlin, C.E.; Brunsell, N.A.; de Oliveira, G.; Crews, T.E.; DeHaan, L.R.; Vico, G. Contrasting Physiological and Environmental Controls of Evapotranspiration over Kernza Perennial Crop, Annual Crops, and C4 and Mixed C3/C4 Grasslands. Sustainability 2019, 11, 1640. [Google Scholar] [CrossRef]
- Sutherlin, C.E.; Brunsell, N.A.; de Oliveira, G.; Crews, T.E.; DeHaan, L.R.; Vico, G. Contrasting Physiological and Environmental Controls of Evapotranspiration over Kernza Perennial Crop, Annual Crops, and C4 and Mixed C3/C4 Grasslands. Sustainability 2019, 11, 1640. [Google Scholar] [CrossRef]
- Tagesson, T.; Fensholt, R.; Cropley, F.; Guiro, I.; Horion, S.; Ehammer, A.; Ardö, J. Dynamics in carbon exchange fluxes for a grazed semi-arid savanna ecosystem in West Africa. Agric. Ecosyst. Environ. 2015, 205, 15–24. [Google Scholar] [CrossRef]
- Tang, J.; Bolstad, P.V.; Ewers, B.E.; Desai, A.R.; Davis, K.J.; Carey, E.V. Sap flux-upscaled canopy transpiration, stomatal conductance, and water use efficiency in an old growth forest in the Great Lakes region of the United States. J. Geophys. Res. Biogeosci. 2006, 111. [Google Scholar] [CrossRef]
- Tang, X.; Ding, Z.; Li, H.; Li, X.; Luo, J.; Xie, J.; Chen, D. Characterizing ecosystem water-use efficiency of croplands with eddy covariance measurements and MODIS products. Ecol. Eng. 2015, 85, 212–217. [Google Scholar] [CrossRef]
- Teixeira, A.d.C.; Bastiaanssen, W.; Ahmad; Moura, M.; Bos, M. Analysis of energy fluxes and vegetation-atmosphere parameters in irrigated and natural ecosystems of semi-arid Brazil. J. Hydrol. 2008, 362, 110–127. [Google Scholar] [CrossRef]
- Vadez, V.; Kholova, J.; Medina, S.; Kakkera, A.; Anderberg, H. Transpiration efficiency: new insights into an old story. J. Exp. Bot. 2014, 65, 6141–6153. [Google Scholar] [CrossRef]
- van Dijke, A.J.H.; Mallick, K.; Schlerf, M.; Machwitz, M.; Herold, M.; Teuling, A.J. Examining the link between vegetation leaf area and land-atmosphere exchange of water, energy, and carbon fluxes using FLUXNET data. Biogeosciences 2020, 17, 4443–4457. [Google Scholar] [CrossRef]
- van Dijke, A.J.H.; Mallick, K.; Schlerf, M.; Machwitz, M.; Herold, M.; Teuling, A.J. Examining the link between vegetation leaf area and land-atmosphere exchange of water, energy, and carbon fluxes using FLUXNET data. Biogeosciences 2020, 17, 4443–4457. [Google Scholar] [CrossRef]
- Varone, L.; Ribas-Carbo, M.; Cardona, C.; Gallé, A.; Medrano, H.; Gratani, L.; Flexas, J. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Viola, R.; Roberts, A.G., Haupt. Tuberization in Potato Involves a Switch from Apoplastic to Symplastic Phloem Unloading, The Plant Cell. 2001.
- Wagle, P.; Xiao, X.; Kolb, T.E.; Law, B.E.; Wharton, S.; Monson, R.K.; Chen, J.; Blanken, P.D.; Novick, K.A.; Dore, S.; et al. Differential responses of carbon and water vapor fluxes to climate among evergreen needleleaf forests in the USA. Ecol. Process. 2016, 5, 1–17. [Google Scholar] [CrossRef]
- Wehr, R.; Saleska, S.R. Calculating canopy stomatal conductance from eddy covariance measurements, in light of the energy budget closure problem. Biogeosciences 2021, 18, 13–24. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167–167. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.B.; Baldocchi, D.D.; Hanson, P.J. Leaf age affects the seasonal pattern of photosynthetic capacityand net ecosystem exchange of carbon in a deciduous forest. Plant, Cell Environ. 2001, 24, 571–583. [Google Scholar] [CrossRef]
- Wood, D.A. Net ecosystem carbon exchange prediction and insightful data mining with an optimized data-matching algorithm. Ecol. Indic. 2021, 124, 107426. [Google Scholar] [CrossRef]
- Wright, G.C.; Rao, R.C.N.; Farquhar, G.D. Water-Use Efficiency and Carbon Isotope Discrimination in Peanut under Water Deficit Conditions. Crop. Sci. 1994, 34, 92–97. [Google Scholar] [CrossRef]
- Xie, J.; Chen, J.; Sun, G.; Zha, T.; Yang, B.; Chu, H.; Liu, J.; Wan, S.; Zhou, C.; Ma, H.; et al. Ten-year variability in ecosystem water use efficiency in an oak-dominated temperate forest under a warming climate. Agric. For. Meteorol. 2016, 218-219, 209–217. [Google Scholar] [CrossRef]
- Xu, L.; Baldocchi, D.D. Seasonal variation in carbon dioxide exchange over a Mediterranean annual grassland in California. Agric. For. Meteorol. 2004, 123, 79–96. [Google Scholar] [CrossRef]
- Yang, J.; A Duursma, R.; De Kauwe, M.G.; Kumarathunge, D.; Jiang, M.; Mahmud, K.; E Gimeno, T.; Crous, K.Y.; Ellsworth, D.S.; Peters, J.; et al. Incorporating non-stomatal limitation improves the performance of leaf and canopy models at high vapour pressure deficit. Tree Physiol. 2019, 39, 1961–1974. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhao, P.; McCarthy, H.R.; Zhao, X.H.; Niu, J.F.; Zhu, L.W.; Ni, G.Y.; Ouyang, L.; Huang, Y.Q. Influence of the decoupling degree on the estimation of canopy stomatal conductance for two broadleaf tree species. Agric. For. Meteorol. 2016, 221, 230–241. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Sun, G.; Fang, X.; Zha, T.; McNulty, S.; Chen, J.; Jin, Y.; Noormets, A. Response of ecosystem carbon fluxes to drought events in a poplar plantation in Northern China. For. Ecol. Manag. 2013, 300, 33–42. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, B.; Huang, Y.; Wang, G. Daily underlying water use efficiency for AmeriFlux sites. J. Geophys. Res. Biogeosciences 2015, 120, 887–902. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, B.; Huang, Y.; Wang, G. The effect of vapor pressure deficit on water use efficiency at the subdaily time scale. Geophys. Res. Lett. 2014, 41, 5005–5013. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




