Submitted:
24 January 2023
Posted:
25 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experiments
2.1. Preparation of composite silver nanoparticles
2.2. Analyzing techniques for composite silver nanoparticles
3. Results and Discussions
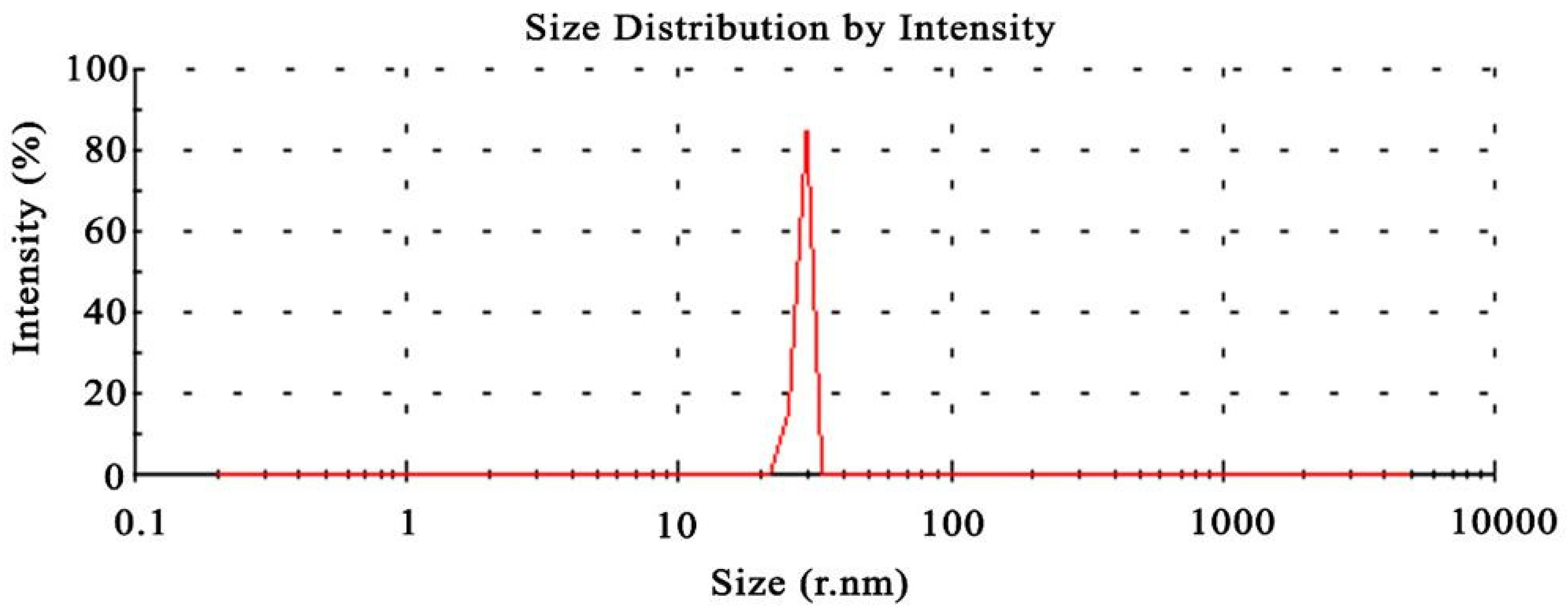
3.1. Particle size of composite silver nanoparticles
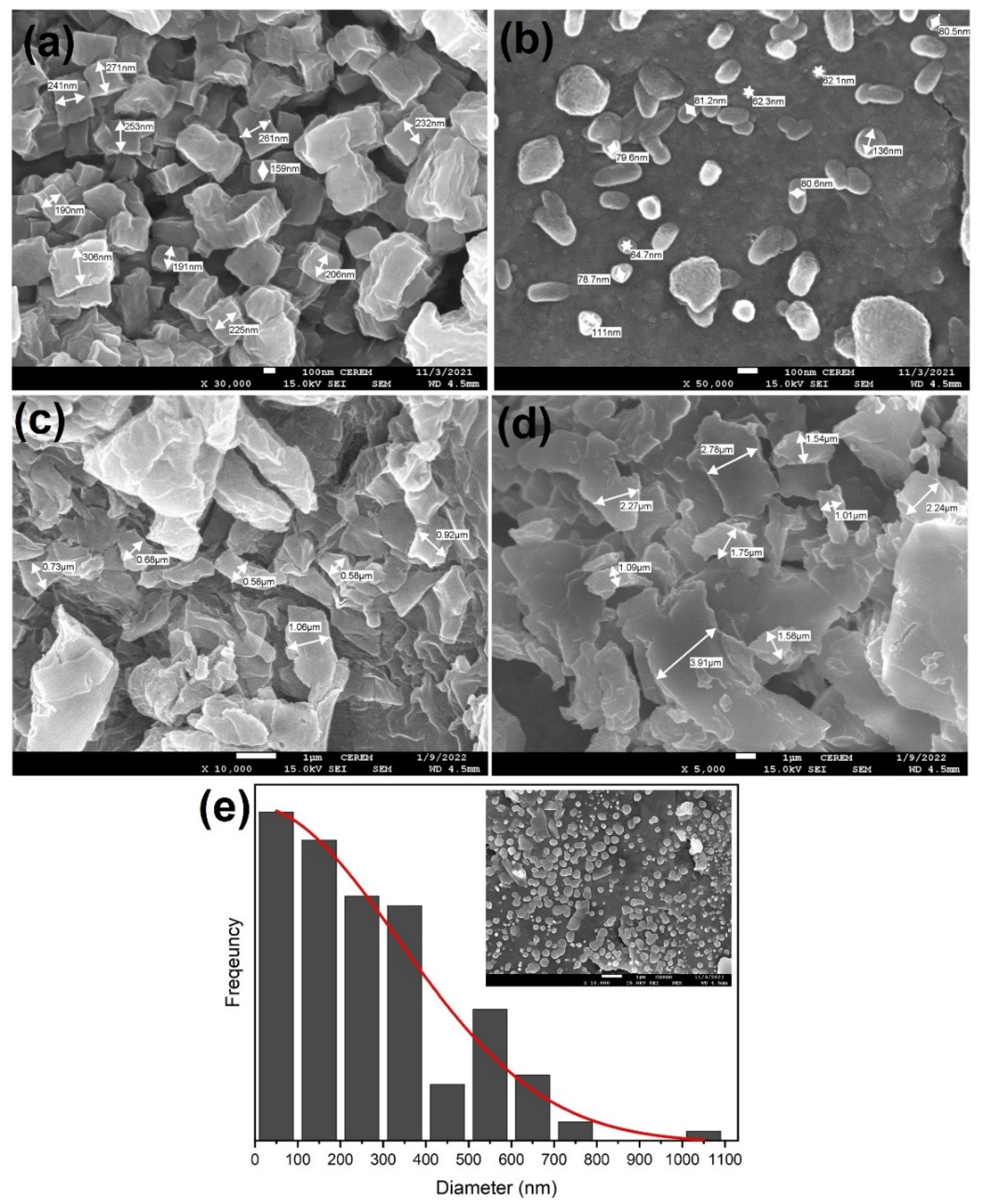
3.2. Morphology of composite silver nanoparticles
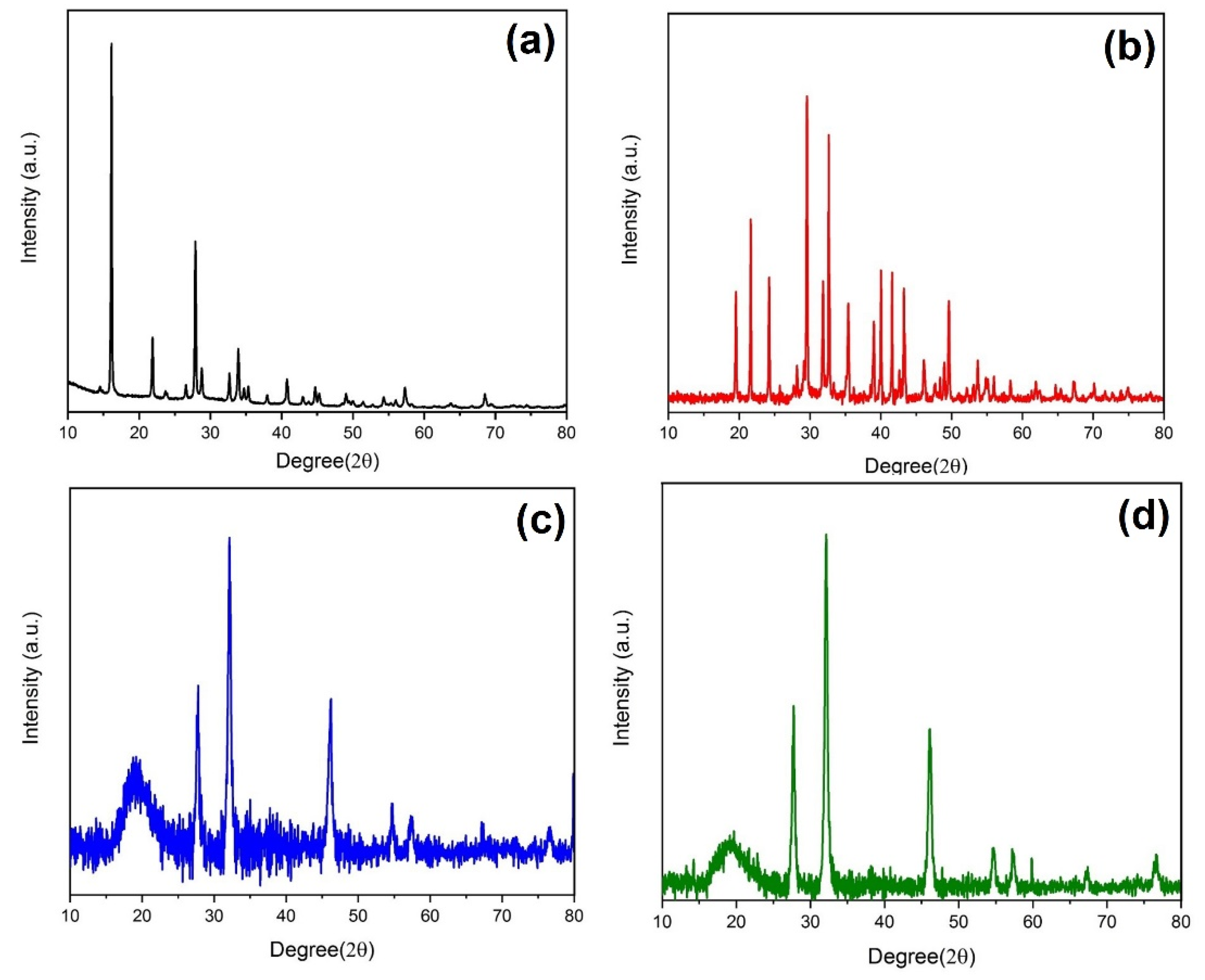
3.3. Spectral properties of composite silver nanoparticles
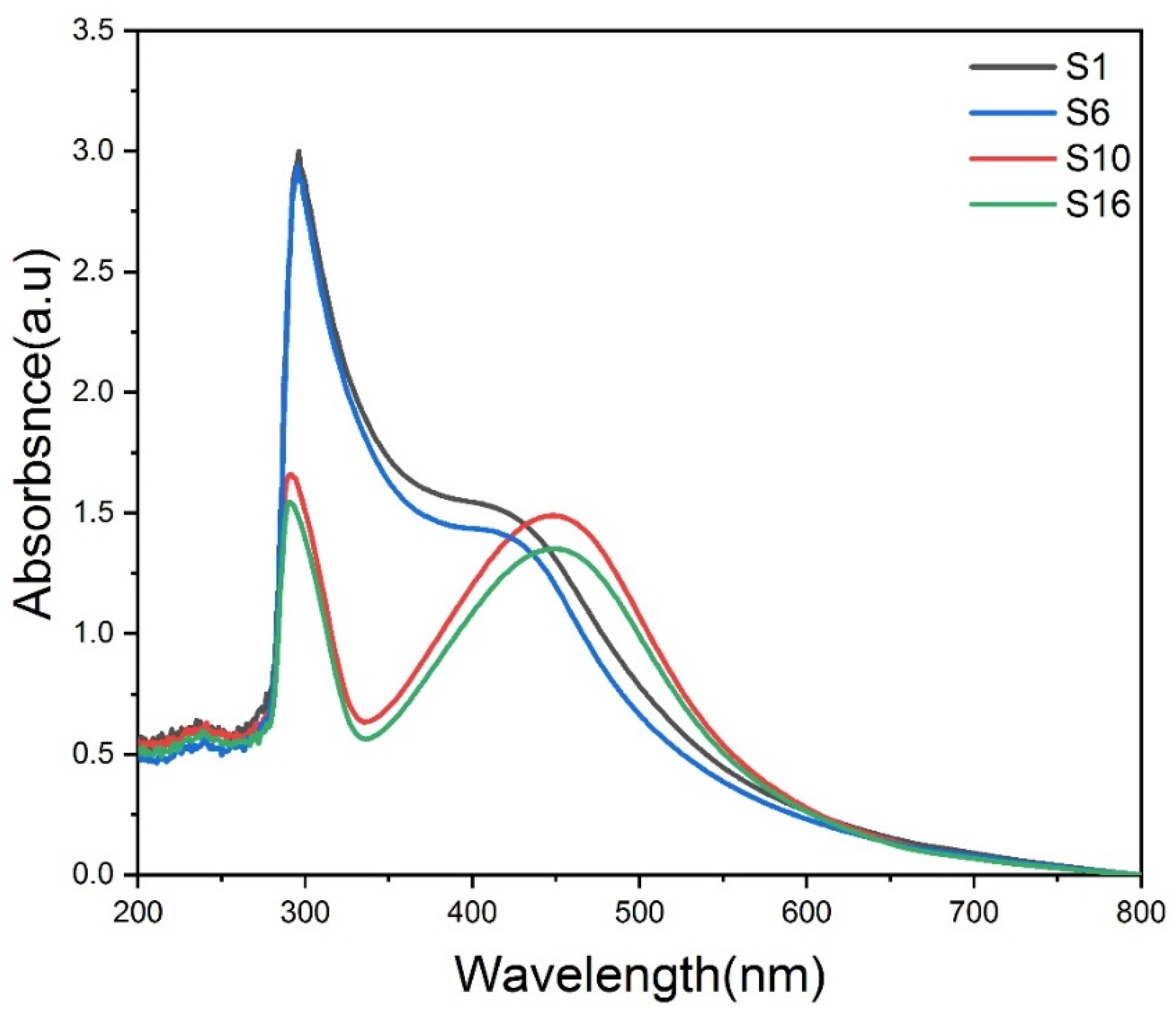
3.4. Optical absorbance and absorption coefficient of composite silver nanoparticles
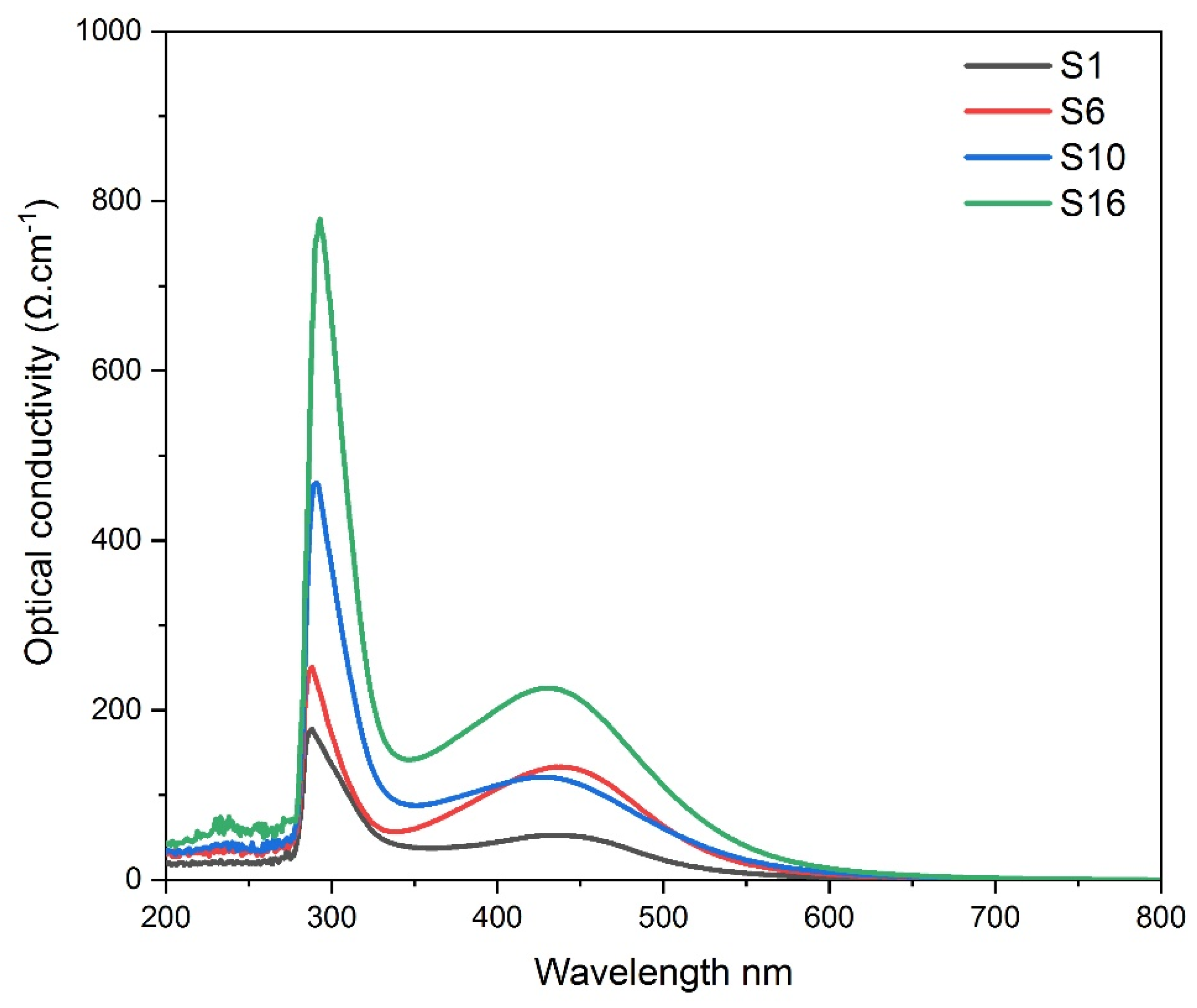
3.5. Optical conductivity of composite silver nanoparticles
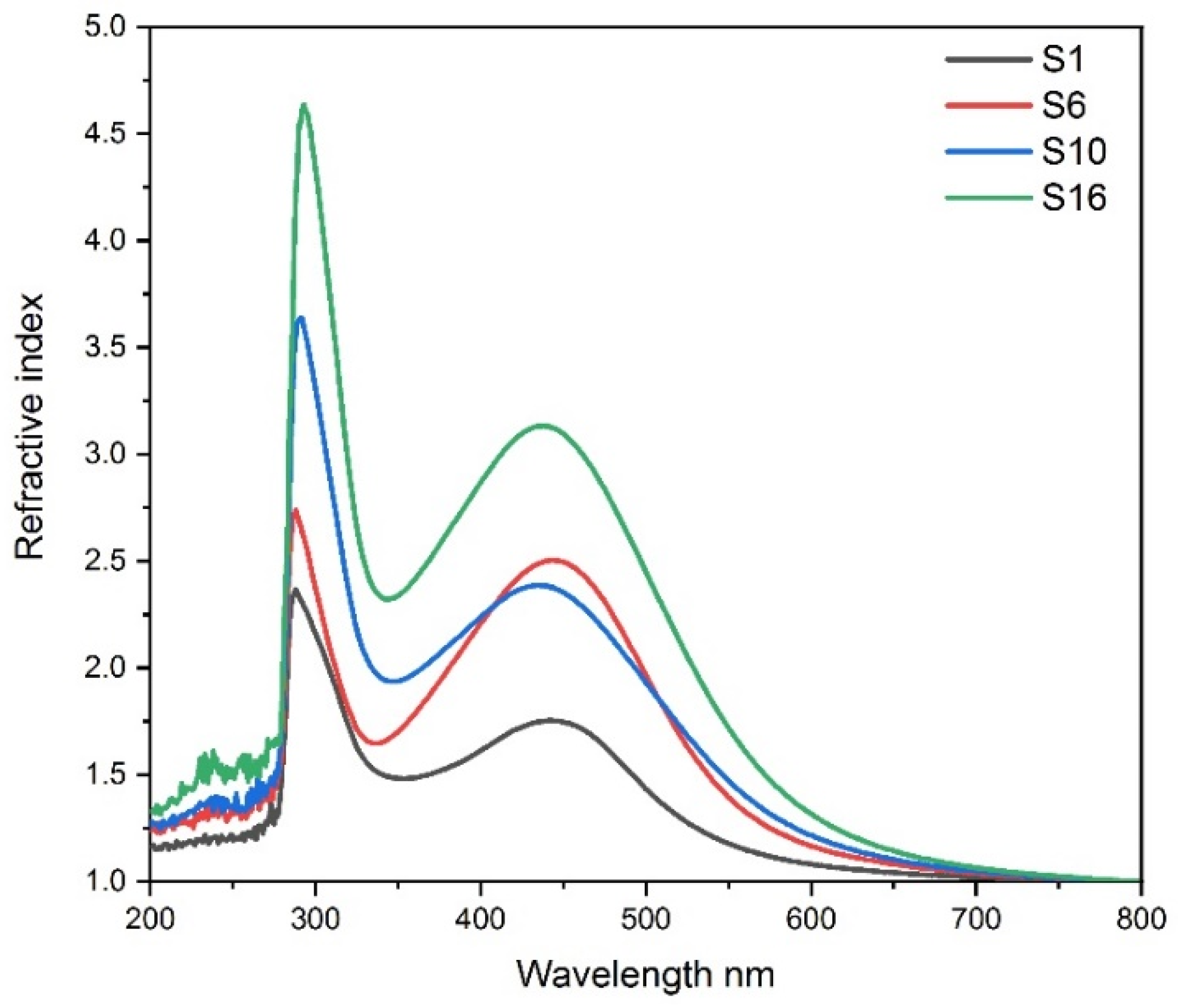
3.6. Variation of refractive index of composite silver nanoparticles
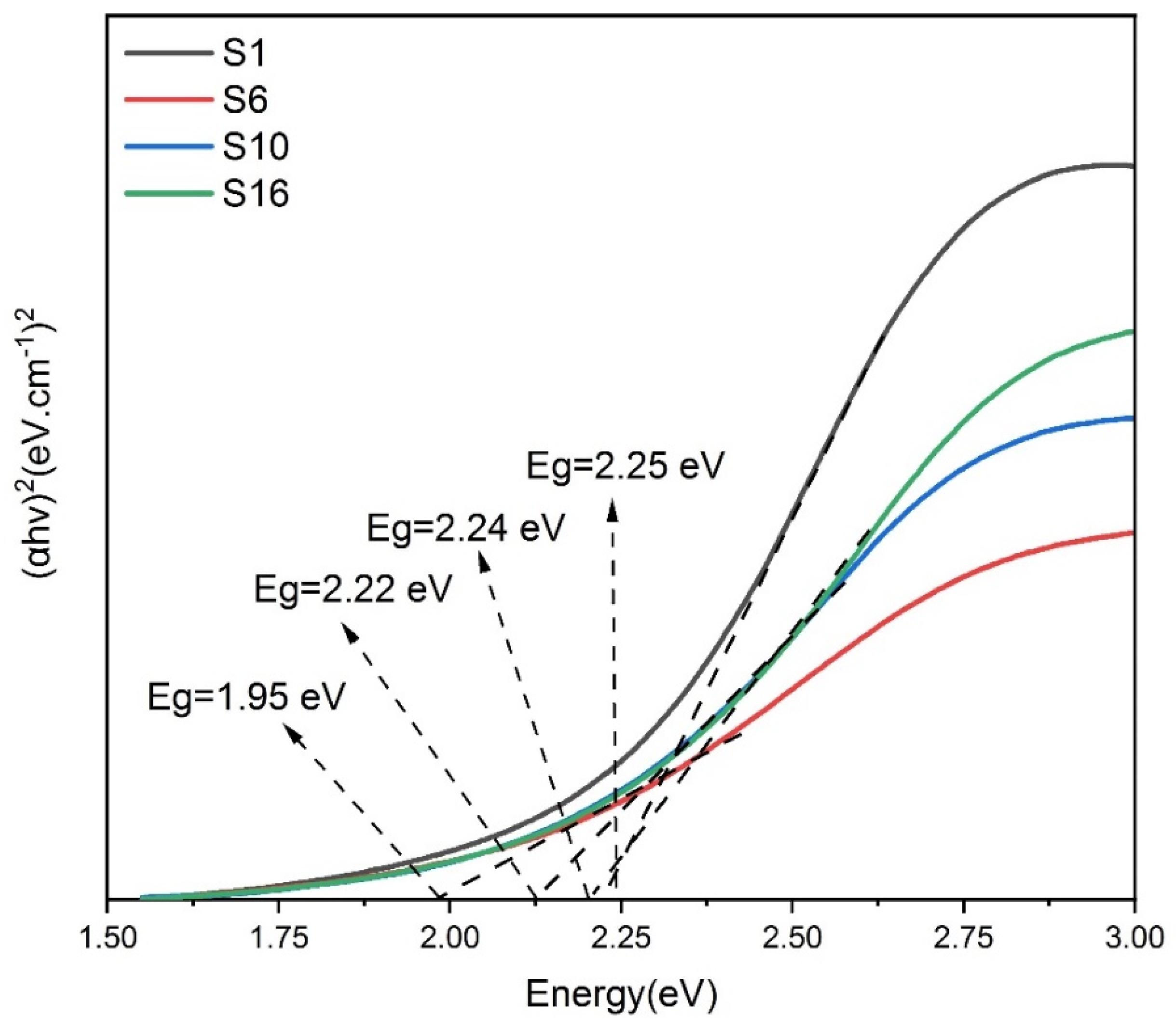
3.7. Band gap energy of composite silver nanoparticles
4. Conclusions
References
- C. Dhand, N. Dwivedi, X.J. Loh, A.N. Jie Ying, N.K. Verma, R.W. Beuerman, R. Lakshminarayanan, S. Ramakrishna, Methods and strategies for the synthesis of diverse nanoparticles and their applications: a comprehensive overview, RSC Adv. 5 (2015) 105003–105037. [CrossRef]
- Ghiuță, D. Cristea, D. Munteanu, Synthesis Methods of Metallic Nanoparticles-an Overview, Bull. Transilv. Univ. Braşov. 10 (2017) 133–140. Available online: http://webbut.unitbv.ro/bulletin/SeriesI/2017/BULETINI/Ghiuta_I.pdf.
- X. Li, H. Xu, Z.S. Chen, G. Chen, Biosynthesis of nanoparticles by microorganisms and their applications, J. Nanomater. 2011 (2011). [CrossRef]
- Ban, I.; Stergar, J.; Drofenik, M.; Ferk, G.; Makovec, D. Synthesis of chromium-Nickel nanoparticles prepared by a microemulsion method and mechanical milling. Acta Chim. Slov. 2013, 60, 750–755. [Google Scholar] [PubMed]
- H.J. Klasen, A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver, Burns. 26 (2000) 131–138. [CrossRef]
- S. Gurunathan, J.H. Park, J.W. Han, J.H. Kim, Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy, Int. J. Nanomedicine. 10 (2015) 4203–4223. [CrossRef]
- W.-R. Li, X.-B. Xie, Q.-S. Shi, H.-Y. Zeng, Y.-S. OU-Yang, Y.-B. Chen, Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli, Appl. Microbiol. Biotechnol. 85 (2010) 1115–1122. [CrossRef]
- P. Mukherjee, A. Ahmad, D. Mandal, S. Senapati, S.R. Sainkar, M.I. Khan, R. Parishcha, P. V Ajaykumar, M. Alam, R. Kumar, M. Sastry, Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis, Nano Lett. 1 (2001) 515–519. [CrossRef]
- S. Chernousova, M. Epple, Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal, Angew. Chemie Int. Ed. 52 (2013) 1636–1653. [CrossRef]
- A.H. Alshehri, M. Jakubowska, A. Młożniak, M. Horaczek, D. Rudka, C. Free, J.D. Carey, Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications, ACS Appl. Mater. Interfaces. 4 (2012) 7007–7010. [CrossRef]
- R. Ma, B. Kang, S. Cho, M. Choi, S. Baik, Extraordinarily High Conductivity of Stretchable Fibers of Polyurethane and Silver Nanoflowers, ACS Nano. 9 (2015) 10876–10886. [CrossRef]
- D. Basak, S. Karan, B. Mallik, Significant modifications in the electrical properties of poly(methyl methacrylate) thin films upon dispersion of silver nanoparticles, Solid State Commun. 141 (2007) 483–487. [CrossRef]
- K. Gupta, P.C. Jana, A.K. Meikap, Optical and electrical transport properties of polyaniline–silver nanocomposite, Synth. Met. 160 (2010) 1566–1573. [CrossRef]
- K. Suganuma, S. Sakamoto, N. Kagami, D. Wakuda, K.-S. Kim, M. Nogi, Low-temperature low-pressure die attach with hybrid silver particle paste, Microelectron. Reliab. 52 (2012) 375–380. [CrossRef]
- B. Bhattarai, I. Chakraborty, B.E. Conn, A. Atnagulov, T. Pradeep, T.P. Bigioni, High-Yield Paste-Based Synthesis of Thiolate-Protected Silver Nanoparticles, J. Phys. Chem. C. 121 (2017) 10964–10970. [CrossRef]
- F.S. Rosarin, S. Mirunalini, Nobel Metallic Nanoparticles with Novel Biomedical Properties, J. Bioanal. Biomed. 03 (2011) 85–91. [CrossRef]
- X. Dai, Q. Guo, Y. Zhao, P. Zhang, T. Zhang, X. Zhang, C. Li, Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing, ACS Appl. Mater. Interfaces. 8 (2016) 25798–25807. [CrossRef]
- Leelavathi, T.U. Bhaskara Rao, T. Pradeep, Supported quantum clusters of silver as enhanced catalysts for reduction, Nanoscale Res. Lett. 6 (2011) 123. [CrossRef]
- D.Y. Zhang, J. Liu, Y.S. Shi, Y. Wang, H.F. Liu, Q.L. Hu, L. Su, J. Zhu, Antifouling polyimide membrane with surface-bound silver particles, J. Memb. Sci. 516 (2016) 83–93. [CrossRef]
- Y. Seo, J. Hwang, J. Kim, Y. Jeong, M.P. Hwang, J. Choi, Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles, Int. J. Nanomedicine. 9 (2014) 4621–4629. [CrossRef]
- M. Riaz, U. Sharafat, N. Zahid, M. Ismail, J. Park, B. Ahmad, N. Rashid, M. Fahim, M. Imran, A. Tabassum, Synthesis of Biogenic Silver Nanocatalyst and their Antibacterial and Organic Pollutants Reduction Ability, ACS Omega. 7 (2022) 14723–14734. [CrossRef]
- K.B. Narayanan, N. Sakthivel, Biological synthesis of metal nanoparticles by microbes, Adv. Colloid Interface Sci. 156 (2010) 1–13. [CrossRef]
- C. Li, Y. Xu, W. Tu, G. Chen, R. Xu, Metal-free photocatalysts for various applications in energy conversion and environmental purification, Green Chem. 19 (2017) 882–899. [CrossRef]
- Conn, J. Guo, B. Yoon, R.N. Barnett, B.M. Monahan, K. Kirschbaum, W.P. Griffith, R.L. Whetten, U. Landman, T.P. Bigioni, Ultrastable silver nanoparticles, Nature. 501 (2013) 399–402. [CrossRef]
- P. Singh, H. Singh, Y.J. Kim, R. Mathiyalagan, C. Wang, D.C. Yang, Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications, Enzyme Microb. Technol. 86 (2016) 75–83. [CrossRef]
- C. Li, Y. Zhang, M. Wang, Y. Zhang, G. Chen, L. Li, D. Wu, Q. Wang, In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window, Biomaterials. 35 (2014) 393–400. [CrossRef]
- Salopek-Sondi, Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria, J. Colloid Interface Sci. 275 (2004) 177–182. [CrossRef]
- M. De, P.S. Ghosh, V.M. Rotello, Applications of nanoparticles in biology, Adv. Mater. 20 (2008) 4225–4241. [CrossRef]
- S.M. Radke, E.C. Alocilja, A high density microelectrode array biosensor for detection of E. coli O157:H7, Biosens. Bioelectron. 20 (2005) 1662–1667. [CrossRef]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells : targeting p53 for anticancer therapy. Int. J. Nanomedicine. 2015, 10, 4203–4223. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A. Ibrahim, O.C.S. Al-Hamouz, T. Laoui, A. Benamor, M.A. Atieh, Fabrication of polysulfone nanocomposite membranes with silver-doped carbon nanotubes and their antifouling performance, J. Appl. Polym. Sci. 134 (2017) 1–12. [CrossRef]
- Ihsanullah, T. Laoui, A.M. Al-Amer, A.B. Khalil, A. Abbas, M. Khraisheh, M.A. Atieh, Novel anti-microbial membrane for desalination pretreatment: A silver nanoparticle-doped carbon nanotube membrane, Desalination. 376 (2015) 82–93. [CrossRef]
- K. Page, R.G. Palgrave, I.P. Parkin, M. Wilson, S.L.P. Savin, A. V. Chadwick, Titania and silver-titania composite films on glass - Potent antimicrobial coatings, J. Mater. Chem. 17 (2007) 95–104. [CrossRef]
- L.E. Rananga, T. Magadzu, Comparative studies of silver doped carbon nanotubes and β-cyclodextrin for water disinfection, Dig. J. Nanomater. Biostructures. 10 (2015) 831–836. Available online: http://www.chalcogen.ro/831_Rananga.pdf.
- A.K. Shukla, J. Alam, M.A. Ansari, M. Alhoshan, M. Alam, A. Kaushik, Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube / polyphenylsulfone nanocomposite membranes, Mater. Chem. Phys. 233 (2019) 102–112. [CrossRef]
- R. Su, Y. Jin, Y. Liu, M. Tong, H. Kim, Bactericidal activity of Ag-doped multi-walled carbon nanotubes and the effects of extracellular polymeric substances and natural organic matter, Colloids Surfaces B Biointerfaces. 104 (2013) 133–139. [CrossRef]
- Ohnuma, K. Iwasaki, T.B. Corporation, Development of n-type silver-nanoparticles-modi fi ed carbon materials doped by triphenylphosphine, MRS Commun. (2017) 1–5. [CrossRef]
- K.M.M. Abou El-Nour, A. Eftaiha, A. Al-Warthan, R.A.A. Ammar, Synthesis and applications of silver nanoparticles, Arab. J. Chem. 3 (2010) 135–140. [CrossRef]
- V.K. Sharma, R.A. Yngard, Y. Lin, Silver nanoparticles: Green synthesis and their antimicrobial activities, Adv. Colloid Interface Sci. 145 (2009) 83–96. [CrossRef]
- W.H.O. (WHO), Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum, 4th ed., World Health Organization, 2017.
- H.G. Gorchev, G. Ozolins, Guidelines for drinking-water quality, 2011. [CrossRef]
- H. Ou-Yang, G. Stamatas, N. Kollias, Spectral Responses of Melanin to Ultraviolet A Irradiation, J. Invest. Dermatol. 122 (2004) 492–496. [CrossRef]
- M. Al Khatib, M. Harir, J. Costa, M.C. Baratto, I. Schiavo, L. Trabalzini, S. Pollini, G.M. Rossolini, R. Basosi, R. Pogni, Spectroscopic Characterization of Natural Melanin from a Streptomyces cyaneofuscatus Strain and Comparison with Melanin Enzymatically Synthesized by Tyrosinase and Laccase, Molecules. 23 (2018). [CrossRef]
| S.No. | AgNo3 ( g) |
DW(distilled water) (ml) |
200 °C 0.5 g AG |
MB(melanin broth) |
|---|---|---|---|---|
| S1 | 0.050 | 100 ml | 0.5 | 50 ml |
| S2 | 0.100 | 100 ml | 0.5 | 50 ml |
| S3 | 0.150 | 100 ml | 0.5 | 50 ml |
| S4 | 0.3 | 100 ml | 0.5 | 50 ml |
| S5 | 0.4 | 100 ml | 0.5 | 50 ml |
| S6 | 0.5 | 100 ml | 0.5 | 50 ml |
| S7 | 0.6 | 100 ml | 0.5 | 50 ml |
| S8 | 0.7 | 100 ml | 0.5 | 50 ml |
| S9 | 0.8 | 100 ml | 0.5 | 50 ml |
| S10 | 0.9 | 100 ml | 0.5 | 50 ml |
| S11 | 1.0 | 100 ml | 0.5 | 50 ml |
| S12 | 1.25 | 100 ml | 0.5 | 50 ml |
| S13 | 1.5 | 100 ml | 0.5 | 50 ml |
| S14 | 1.75 | 100 ml | 0.5 | 50 ml |
| S15 | 2 .0 | 100 ml | 0.5 | 50 ml |
| S16 | 2.25 | 100 ml | 0.5 | 50 ml |
| S17 | 2.50 | 100 ml | 0.5 | 50 ml |
| S18 | 2.75 | 100 ml | 0.5 | 50 ml |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




