1. Introduction
Lumbar spinal facet joints were first suggested in the medical literature as a source of lower back and lower extremity pain in 1941 [
5]. Since then, the so-called “facetogenic back pain” has become a widely accepted diagnosis, though still controversial in the literature [
5,
6,
7,
8,
9,
10,
11]. The most substantial support comes from investigations reporting successful back pain relief following peri-articular joint injections [
12,
13]. Estimates of lumbar facet joint pain prevalence based on single diagnostic blocks have ranged from 7.7% to 75% among patients reporting back pain [
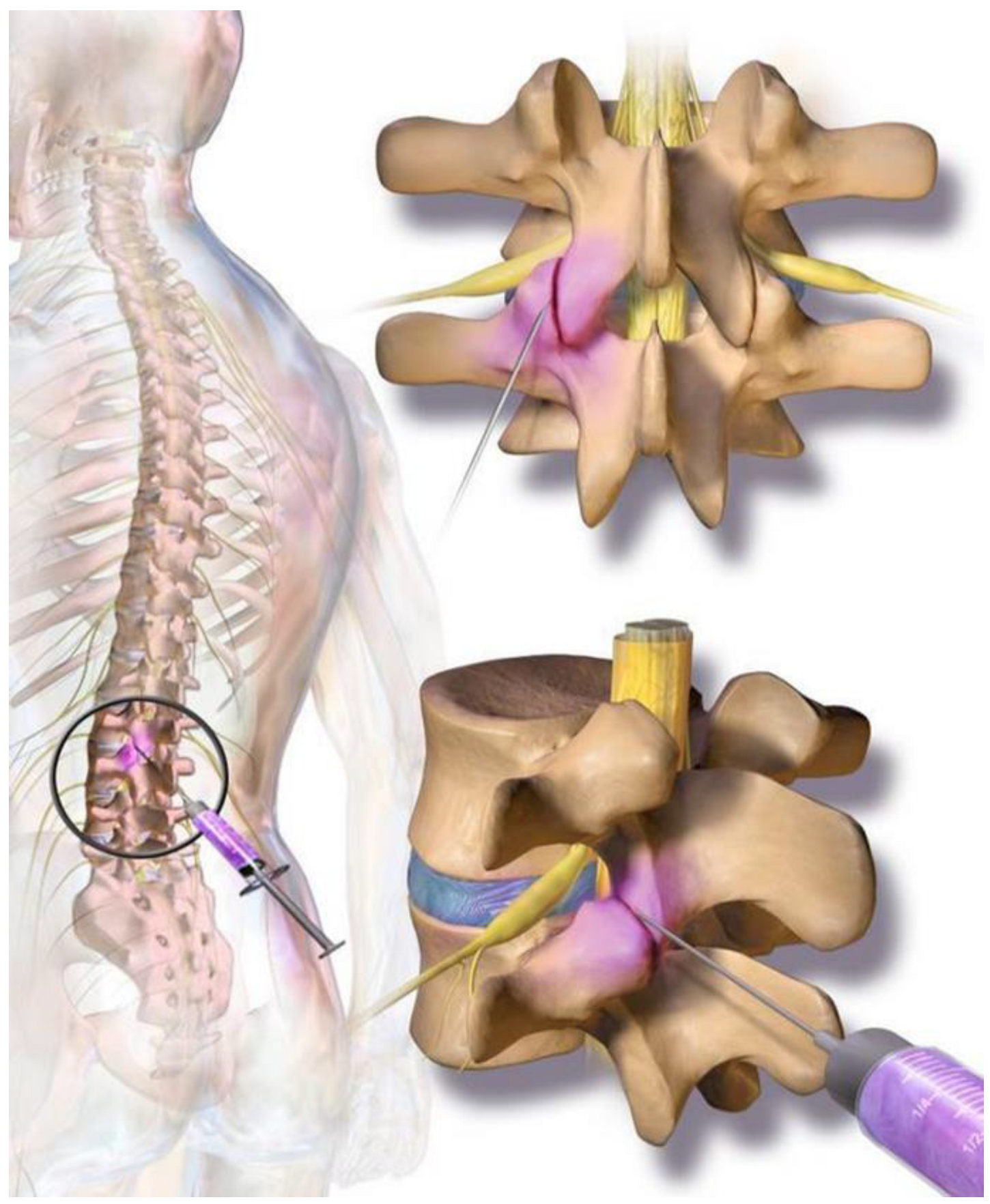
14]. The facet joint syndrome is a degenerative process of the facet joint’s cartilage following degeneration of an intervertebral disc that leads to height loss of the segment resulting in a mechanically induced overload and arthrosis of the respective facet joints (
Figure 1). These small joints are made for flexibility but not for weight bearing. Mechanically-induced chronic weight overload finally results in a chronic inflammatory process involving the immune system, producing local inflammatory reactions with the synthesis of several pro-inflammatory cytokines and metalloproteinases [
15]. Due to the inflammatory nature of the disease, local injection of glucocorticoids into the affected joint has become a standard treatment option. However, several studies suggest that such injections represent no long-term treatment for patients with chronic back pain [
16]. Recently it could be shown that interactions of human bone marrow-derived stem cells (MSCs) can limit and mitigate the inflammatory responses in the peri-articular fat by promoting anti- inflammatory pathways [
17].
Bone Marrow derived MSCs are harvested by puncture of the iliac crest. This procedure is likely accompanied by some risks to the patient, such as long-term damage to the regenerative hematopoietic potential of the bone marrow- after the removal of hundreds of millions of valuable bone marrow cells. About 99% of the cells removed are hematopoietic progenitors, less suitable for tissue renewal outside the hematopoietic system. It seems inefficient to recover a small number of actual pluripotent stem cells to damage the pool of valuable bone marrow cells primarily as it is known that the number of vital bone marrow cells declines with increasing age.
With the InGeneronTM process, many stem cells can be recovered from adipose tissue [
3]. These stem cells from adipose tissue provide three scientifically proven functions: anti-inflammatory effects, antiapoptosis, tissue repair, renewal, and re-placement [
18]. These ADRCs can transform into any somatic cell by appropriately exchanging genetic information within the so-called "micro-environment" of the tissue or organ they are placed in [
1]. Consequently, local tissue damage can be treated by injecting stem cells at the site needing repair. Clinical applications have shown that tissue of all three germ lines can be treated with stem cells [
2,
25,
26]. In degenerative diseases of joints, damage to functional tissue induces an inflammatory process, resulting in pain. It has been shown that painful chronic inflammatory disorders of the musculoskeletal system can successfully be treated by stem cell injection [
19]. Stem cells, in general, often called MSCs in an inflammatory environment, can influence the immune response by altering cytokine secretion from macrophages, dendritic and T-cell subsets, resulting in a shift from a pro-inflammatory to an anti-inflammatory environment. Successful treatment of degenerative inflammatory processes of the musculoskeletal system, such as facet joints, knee, or shoulder, with injections of adipose tissue-derived stem cells, are well reported in human medicine [
20,
21].
Adipose Tissue-Derived Regenerative Cells obtained with the InGeneron process contain many so-called vascular-associated pluripotent stem cells [
1,
2]. These cells can be recovered within about 20 minutes from a small amount of fatty tissue, without significant harm to the patient.
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
Patients aged 18-80 years with suspected facet joint syndrome and ongoing symptoms of more than one-year duration – despite standard therapeutic measures - were screened for inclusion in the study. A Fluoroscopically controlled infiltration of the tissue next to the facet joints by 1ml (5 mg/ml) ropivacaine was performed in all patients. This was done to differentiate the source of pain from other causes. Temporary relief of the patient's pain, an analysis of the patient's MRI findings, and a thorough clinical examination were used to establish the diagnosis of "Facet joint Syndrome" before the patient could be included in the study.
Exclusion criteria were: other causes of chronic back pain such as acute disc-related pain, spinal stenosis, osteonecrosis, evidence of a malignant neoplasm in the last 24 months, a history of basal cell carcinoma, chronic skin diseases, connective, metabolic, or skin diseases, evidence of an active infection, immunosuppressive medication, renal insufficiency (creatinine > 1, 8 mg/dl) or liver failure (GOT, GPT > 2x typical values, bilirubin > 2 mg/dl). Thirty-seven patients met the inclusion criteria (age 31-78 years, mean age 62.5 years) and were enrolled in the study.
2.2. Tissue preparation and treatment
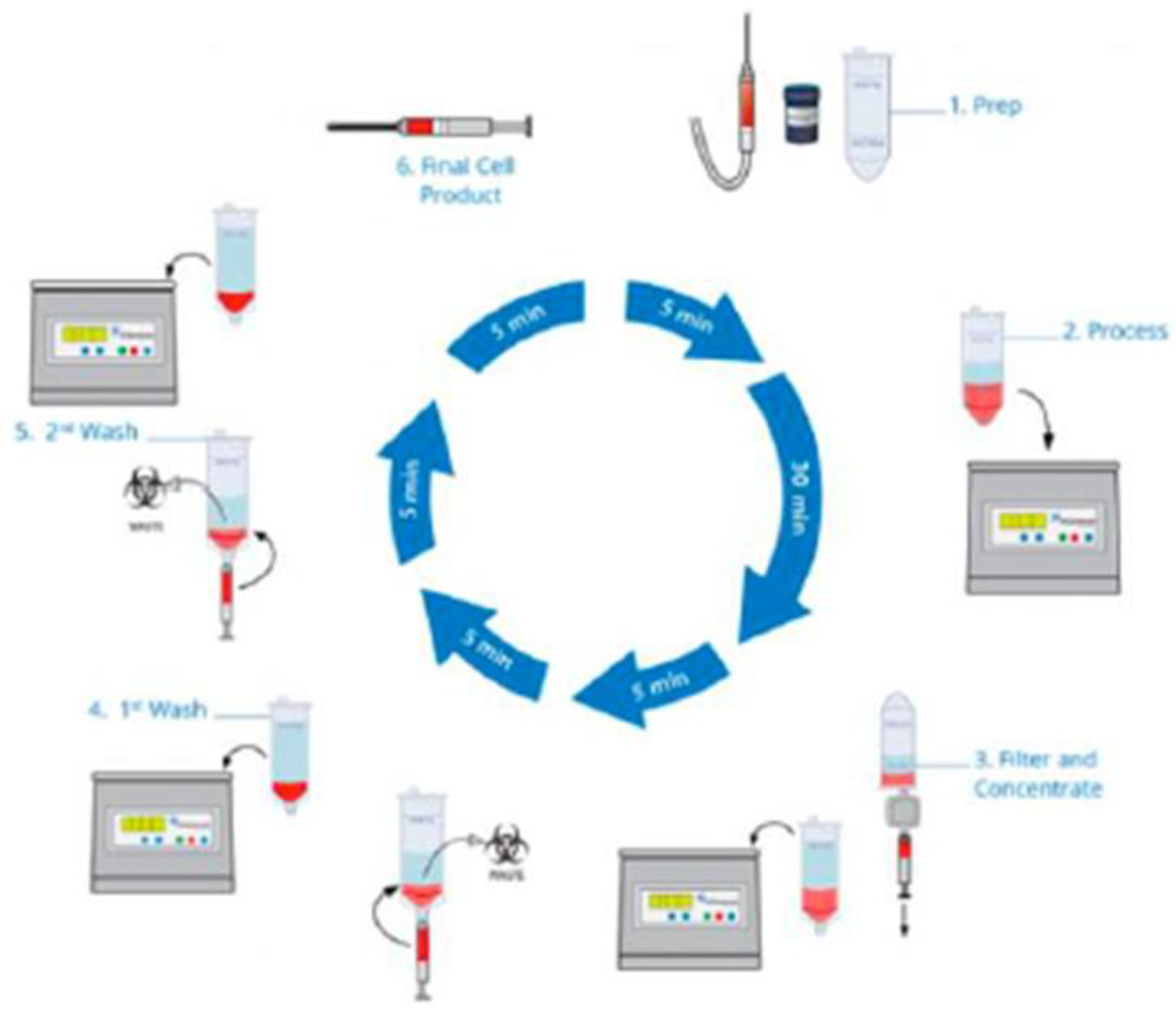
A Mini-Liposuction was performed under local anesthesia with additional conscious sedation affected by propofol iv injection under the supervision of an experienced anesthesiologist. 50-100 ml of abdominal fat tissue was removed by this mini-liposuction and processed using a commercially available system. To recover stem cells from adipose tissue, we use the CE-certified Transpose RT™ system from InGeneron GmbH. The removed adipose tissue sample was transferred into InGeneron’s processing tube, and Matrase™ enzymatic reagent was added to the sample before it was processed in the Transpose® Tissue Processing Unit. During the first run, the extracellular enzymatic degradation by the specific Matrase TM enzyme liberates the regenerative cells from their surrounding matrix. Three subsequent filtering and washing steps are performed to separate and purify the regenerative cells from fat and adipocytes, remaining enzymes, and cell debris. Within 60 minutes, purified regenerative stem cells recovered from the patient’s abdominal fat tissue were ready for immediate use in the same patient as an autologous transplant
Figure 1.
The cellular preparation of these fresh, autologous ADRCs was injected under fluoroscopic control para-articular on both sites of the individually affected facet joints (L2/ to L5 and L5/S1) within the same session of fat tissue recovery, preparation of the cells, and injection into the patient. The fat removal to injection procedure was typically finished in two to three hours (
Figure 2).
2.3. Follow-up
As part of the follow-up, the Visual Analogue Scale (VAS) for back and leg pain and the Oswestry Disability Index (ODI) were recorded before treatment and postoperatively at one week, one year, and five years after the treatment. Kind and type, severity and duration of any adverse events were recorded at each postoperative visit.
3. Results
3.1. Preoperative symptoms
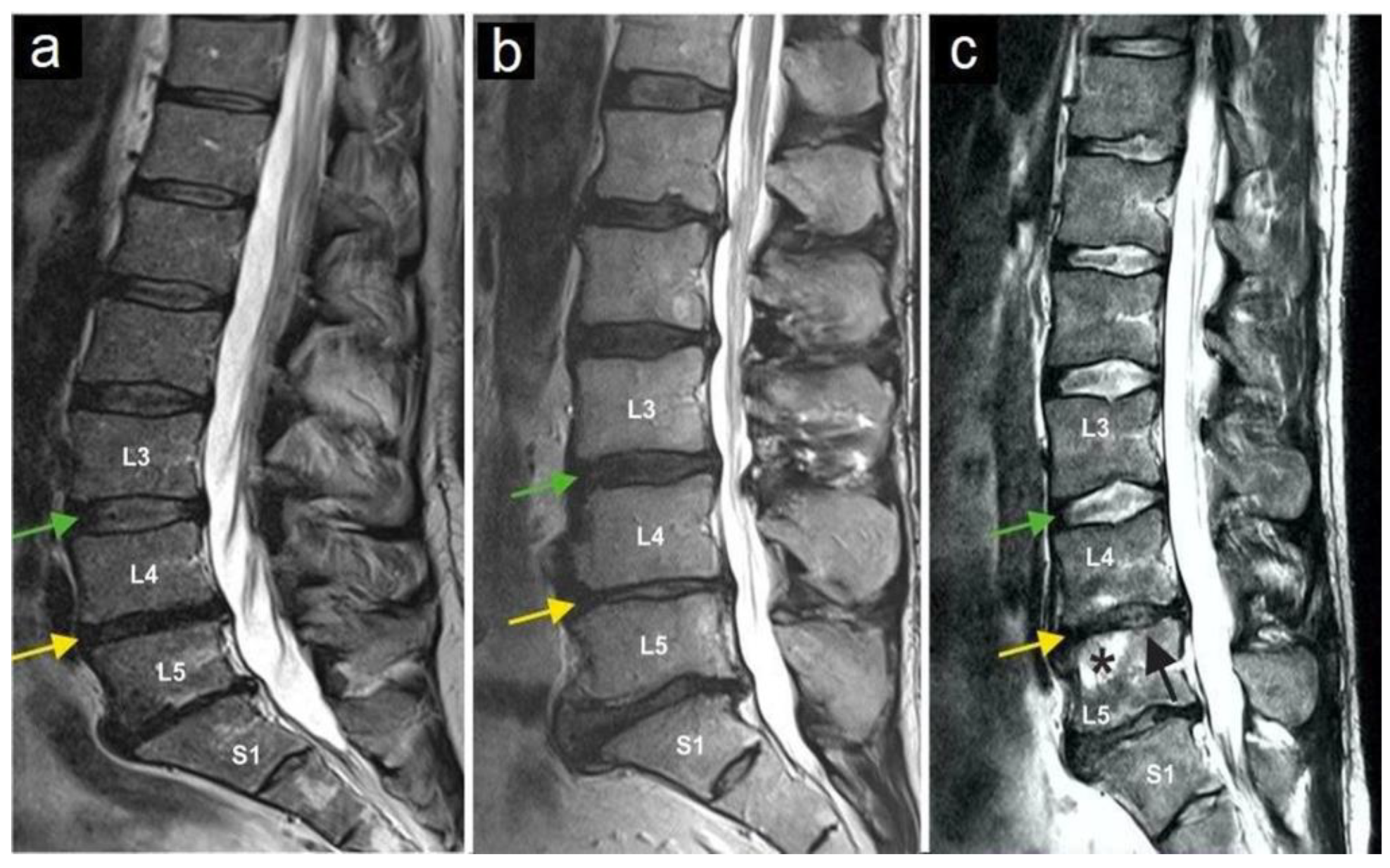
The most common preoperative symptom was local back pain (in 100% of cases). Before treatment, none of the patients had a motor deficit. One patient had suffered from a sensory deficit at L5 on the right side (2.7%) after six previous operations on the lumbar spine. Only lumbar pain syndromes were included in the evaluation. In the preoperative MRI, nerve root compression was ruled out in all patients, and all patients showed degenerative changes in facet joints. In 7 patients (18,9%), there were radiological signs of osteochondrosis Modic I and II (
Figure 3).
3.2. Pain Levels and Oswestry Disability Index
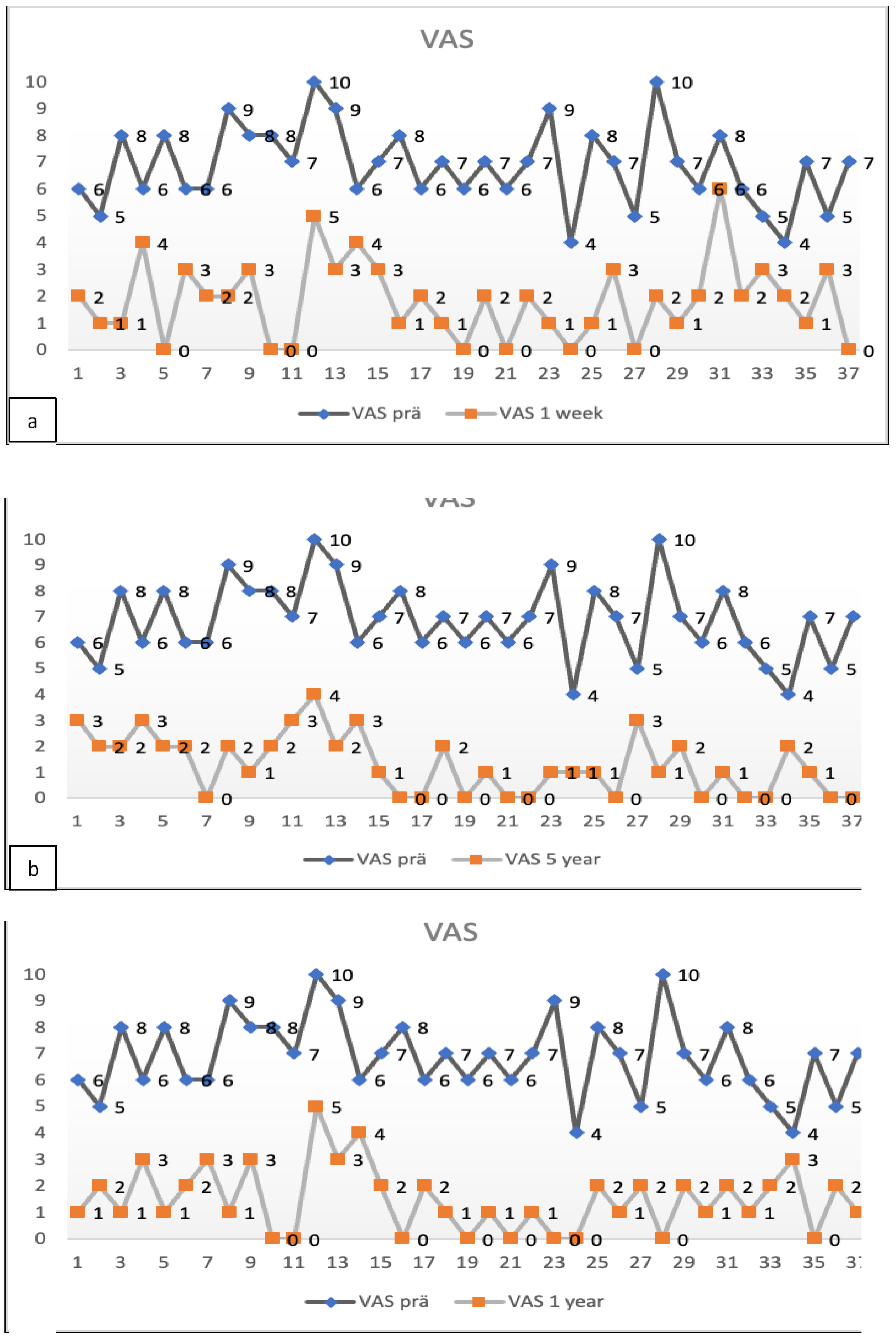
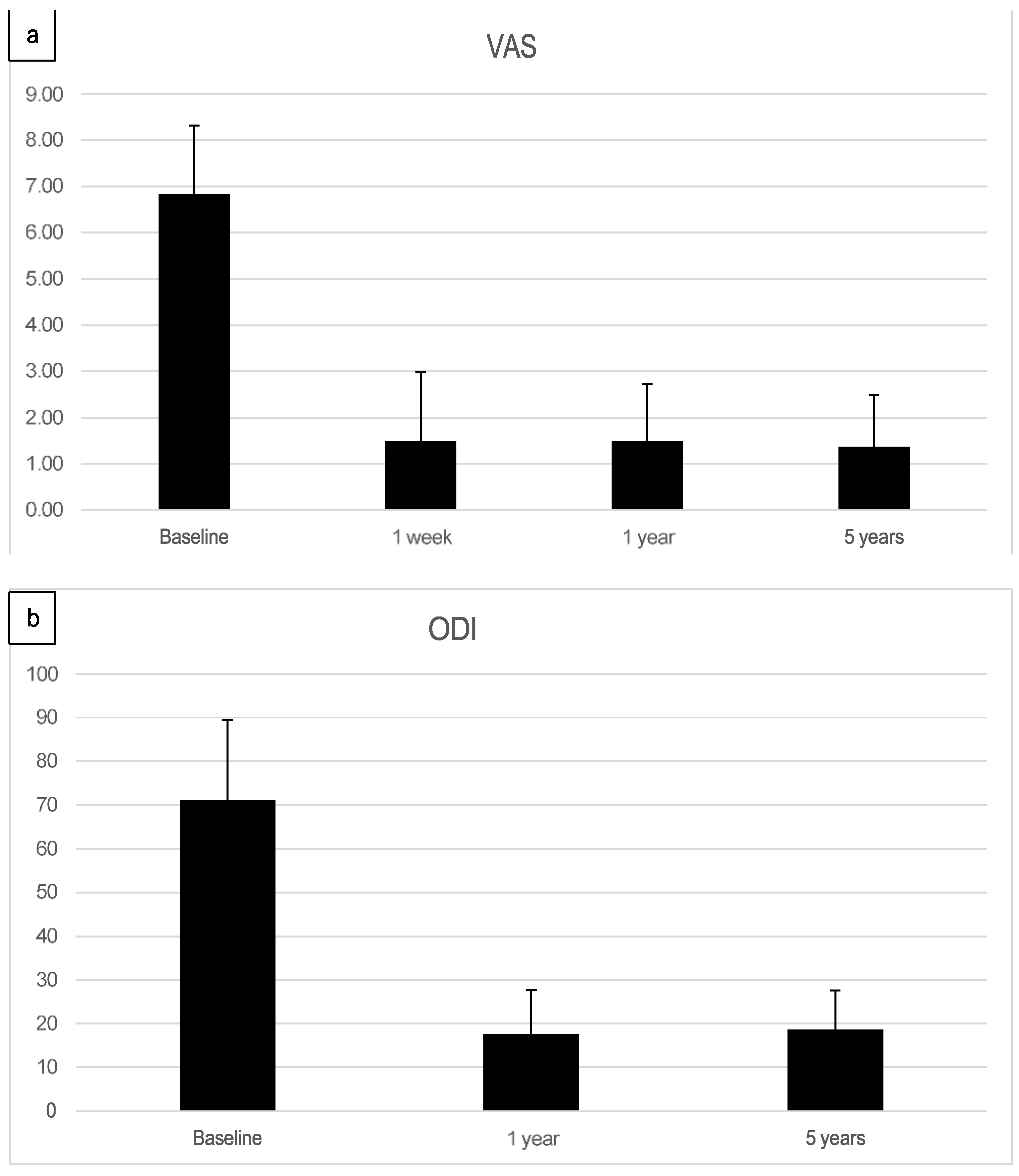
The preoperative pain level measured by the Visual Analog Scale means level was VAS 6.8 with an individual range of 5-10 (on a scale of 0= no pain, 10=maximum pain), and the Oswestry Disability Index (ODI) was assessed at 71.05% (range 43-91). After one week, patients already reported an improvement reflected by a decreased Visual Analog Scale pain level. At the one-year follow-up (mean 13.2 months, range 12-16 months), the mean VAS level was 1.5, and the mean ODI was 17.5%. At the five-year follow-up (mean 61.7 months), the mean VAS level was 1.4, and the mean ODI was 18.7%. Every patient reported improved VAS pain after treatment with ADRCs compared to the baseline (
Figure 4 and
Figure 5). This result was observed in the short term (one week and one month) and in the longer term after five years of follow-up.
3.3. Complications
After liposuction, one patient developed a relatively sizeable subcutaneous hematoma of about 10 x 15 cm at the liposuction site. This condition was treated conservatively. It caused local pain for 17 days and resorbed spontaneously without further consequences. There were no other minor or severe complications, particularly no infections; no local or systemic minor or significant adverse events related to the procedure.
4. Discussion
Around 70% of all people will suffer at least once from severe lower back pain, as about 25% of all people suffer from chronic lumbago. Causal therapy is unknown, and regular use of NSAIDs often leads to unfavorable comorbidities and is associated with considerable socio-economic costs. In the current study, we report initial clinical results of ADRC treatment and five-year follow-up in patients with facet joint syndrome.
In the context of degenerative lumbar spine diseases, pain is primarily due to inflammatory reactions resulting from degenerative changes. Inflammatory cytokines have been demonstrated in the facet joint [
7] to produce arthropathic changes in degenerative lumbar spine disease. As a result, pain is linked to chemical factors associated with inflammatory responses and cytokines. This theory corroborates the better clinical effects of nonsteroidal anti-inflammatory drugs and corticosteroids over opioids or other analgesics [
12]. The immune-modulatory impact of stem cells is, therefore, of great interest for the prevention of inflammatory reactions in degenerative diseases of the musculoskeletal system. Successful treatment has also been reported with experimental autoimmune disorders such as collagen-induced arthritis and multiple sclerosis. However, conflicting results on how immune modulation is achieved by stem cells are still discussed [
10]. Our study used adipose tissue stem cells (ADSCs) because several prior studies have found a significant qualitative difference between bone marrow stem cells and adipose tissue stem cells. The main difference is the significantly higher number of vascular-associated pluripotent stem cells found in adipose tissue, plus the more facilitated access to adipose tissue compared to bone marrow [
2,
3]. Harvesting a small amount of fatty tissue less damages the patient than removing millions of valuable bone marrow cells. Bone marrow yields a relatively low number of genuinely regenerative cells. Culturing these cells (MSCs) before use in clinical applications is custom, leading to gene expression changes [
22]. Adipose tissue-derived stem and regenerative cells can be isolated and administered in large numbers at the “point of care” without cell expansion/culturing [
23]. These non-engineered cells also lead to high clinical safety and efficacy [
24,
25,
26]
Our data suggest an initial anti-inflammatory impact of the cells used. We also clinically observed an acute anti-inflammatory effect of the ADSCs, evidenced by the first clinical improvement often seen already 48 hours after treatment. This result suggests an additional therapeutic benefit for other degenerative diseases of the musculoskeletal system. Like regulatory T cells (TREG), ADSCs can migrate to joints where they can act locally within the inflamed synovium to reduce immune cell proliferation and function through the secretion of soluble inhibitory factors. They can also systematically suppress the host immune response through a shift in T1/T2 cell balance, suggesting that ADSC-induced immune modulation is not only mediated by a single mechanism indicating critical therapeutic applications well beyond the field of degenerative joint disease. Further studies should help better understand stem cells' underlying mechanisms of immune modulation and how stem cells derived from adipose tissue exert the same function in a new location as they do in the initial site of adipose tissue harvested from.
5. Conclusions
The current study is the first publication in the scientific literature of a 5-year follow-up long-term assessment evaluating the treatment of chronic facet joint syndrome with autologous adipose tissue-derived stem cells. All patients in this study served as their internal paired control. The authors' results warrant further investigation of ADRCs for facet joint syndrome treatment. Future randomized prospective controlled trials assessing the safety and efficacy of ADRC treatment in comparison to the best-known standard of care interventions should be conducted to corroborate the results of this long-term observational study.
Author Contributions
Conceptualization, EA. and R.R.; methodology, E.A.; software, RR.; validation EA. and R.R.; formal analysis, EA. and R.R investigation, J.T., EA. and R.R.; writing—original draft preparation, R.R. writing—review and editing, EA. and R.R.
Funding
This research was funded by Isarkliniken GmbH in Munich and by the Internationale Stiftung Medizin und Wissenschaft in Munich.
Institutional Review Board Statement
The study was approved by Decisions of The Freiburg International Ethics Kommitee (FEKI ) and the Ethics Kommitee of the Bavarian Physician Association.
Freiburger Ethik Kommision International: Votum Code 013/1372; Autologe Zellanreicherung zur Behandlung der Arthrose
Freiburger Ethik Kommision International: Votum Code 016/1252; Safety and Efficacy of Injection of adipose-derived mesenchymal stem cells (ADRCs) in patients suffering from osteoarthritis of facet joints
Ethik Kommision der Bay Landesärzte Kammer: Datenerhebung zur postoperativen Erlebnisqualität in der Wirbesäulenchirurgie. 14. Okt 2022
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study including to publish the clinical data and this paper.
Acknowledgments
Isarkliniken GmbH in Munich and by the Internationale Stiftung Medizin und Wissenschaft in Munich.
Conflicts of Interest
Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results must be declared in this section.
The funders had no role in the design of the studyin the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The author RR declares no conflict of interest. EA is Managing Director of Isarklinik II AG, and Executive Chair of InGeneron Inc. JT is a former Employee of Isarkliniken GmbH”.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- Alt EU, Winnier G, Haenel A, et al. Towards a Comprehensive Understanding of UA-ADRCs (Uncultured, Autologous, Fresh, Unmodified, Adipose Derived Regenerative Cells, Isolated at Point of Care) in Regenerative. Cells. 2020;9(1097). [CrossRef]
- Alt EU, Schmitz C, Bai X. Perspective: Why and How Ubiquitously Distributed, Vascular- Associated, Pluripotent Stem Cells in the Adult Body are the Next Generation of Medicine. Cells. 2021;10(9). [CrossRef]
- Winnier GE, Valenzuela N, Peters-Hall J, Kellner J, Alt C, Alt EU. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS One. 2019;14(9). [CrossRef]
- Goldthwait JE. The Lumbo-Sacral Articulation; An Explanation of Many Cases of “Lumbago,” “Sciatica” and Paraplegia. Bost Med Surg J. 1911;164(11):365-372. [CrossRef]
- Badgley, CE. The Articular Facets in Relation to Low-Back Pain and Sciatic Radiation. Journal of Bone and Joint Surgery, 1941 23, 481-496.
- Carrera GF, Haughton VM, Syvertsen A, Williams AL. Computed tomography of the lumbar facet joints. Radiology. 1980;134(1):145-148. [CrossRef]
- Helbig T, Lee CK. The lumbar facet syndrome. Spine (Phila Pa 1976). 1988;13(1):61-64. [CrossRef]
- Manchikanti L, Boswell M V., Singh V, Pampati V, Damron KS, Beyer CD. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15. [CrossRef]
- Manchikanti L, Pampati V, Fellows B, Bakhit CE. The diagnostic validity and therapeutic value of lumbar facet joint nerve blocks with or without adjuvant agents. Curr Rev Pain. 2000;4(5):337-344. [CrossRef]
- Nachemson AL. Newest knowledge of low back pain. A critical look. Clin Orthop Relat Res. 1992;279(279):8-20. [CrossRef]
- Raskin SP. Degenerative changes of the lumbar spine: assessment by computed tomography. Orthopedics. 1981;4(2):186-194. [CrossRef]
- Lewinnek GE, Warfield CA. Facet joint degeneration as a cause of low back pain. Clin Orthop Relat Res. 1986;213(213):216-222. [CrossRef]
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976). 1994;19(10):1132-1137. [CrossRef]
- Dreyer SJ, Dreyfuss PH. Low back pain and the zygapophysial (facet) joints. Arch Phys Med Rehabil. 1996;77(3):290-300. [CrossRef]
- Igarashi A, Kikuchi S, Konno S, Olmarker K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine (Phila Pa 1976). 2004;29(19):2091- 2095. [CrossRef]
- Carette S, Marcoux S, Truchon R, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991;325(14):1002-1007. [CrossRef]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815-1822. [CrossRef]
- Sadat S, Gehmert S, Song YH, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363(3):674-679. [CrossRef]
- Furia JP, Lundeen MA, Hurd JL, et al. Why and how to use the body’s own stem cells for regeneration in musculoskeletal disorders: a primer. J Orthop Surg Res. 2022;17(1):1-7. [CrossRef]
- Hurd JL, Facile TR, Weiss J, et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: A prospective, randomized, controlled first-in-human. J Orthop Surg Res. 2020;15(1):1-18. [CrossRef]
- Alt E, Rothoerl R, Hoppert M, et al. First immunohistochemical evidence of human tendon repair following stem cell injection: A case report and review of literature. World J Stem Cells. 2021;13(7):944-970. [CrossRef]
- Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2007;36(7):613-622. [CrossRef]
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724-2752. [CrossRef]
- Lalu M, McIntyr M, Pugliese C, Fergusson D, Winston B, Marshall JC, Granton J, Stewart DJ, for the Canadian Critical Care Trials Group. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLOS One Oct 2012 1-21. 2012. [CrossRef]
- Tomasz Trafny, Silvio Spiri (eds), Bioethics and Research on Adult Stem Cells, Vol. I: Towards Therapeutic Solutions, IF press, Rome 2020, ISBN 978-88-6788-202-1.
- Schmitz C, Alt C, Azares A, Pearce D, Facile T, Furia JP, Maffulli N, Huang C, Alt EU:Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System DoesNot Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity. Cells 2023, 12, 30. [CrossRef]
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).