Submitted:
30 January 2023
Posted:
02 February 2023
You are already at the latest version
Abstract
Keywords:
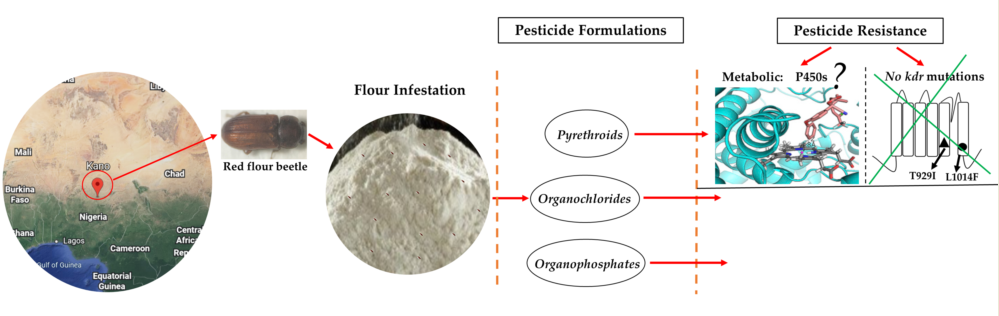
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Beetles
2.2. Morphological Identification of the Beetles
2.3. Insecticides Susceptibility Filter Paper Bioassays
2.4. Investigation of the Role of Metabolic Resistance Using Synergist Bioassay
2.5. Investigation of the Role of Target-Site Pyrethroid/DDT Insensitivity Resistance Mutations
2.6. Molecular Identification of the Beetles
2.7. Data Analysis
3. Results
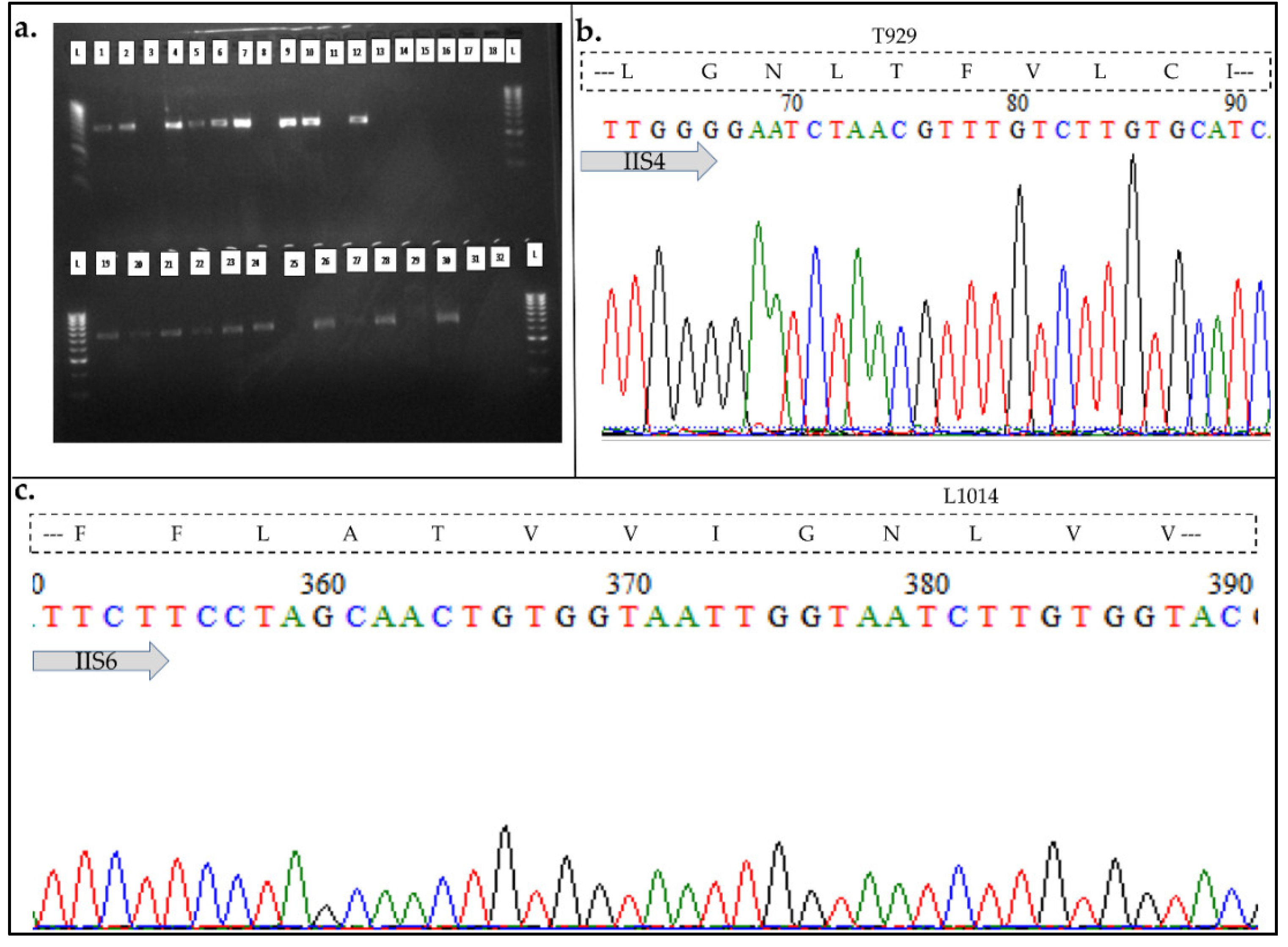
3.1. Morphological and Molecular Identification of Beetles to Species Level
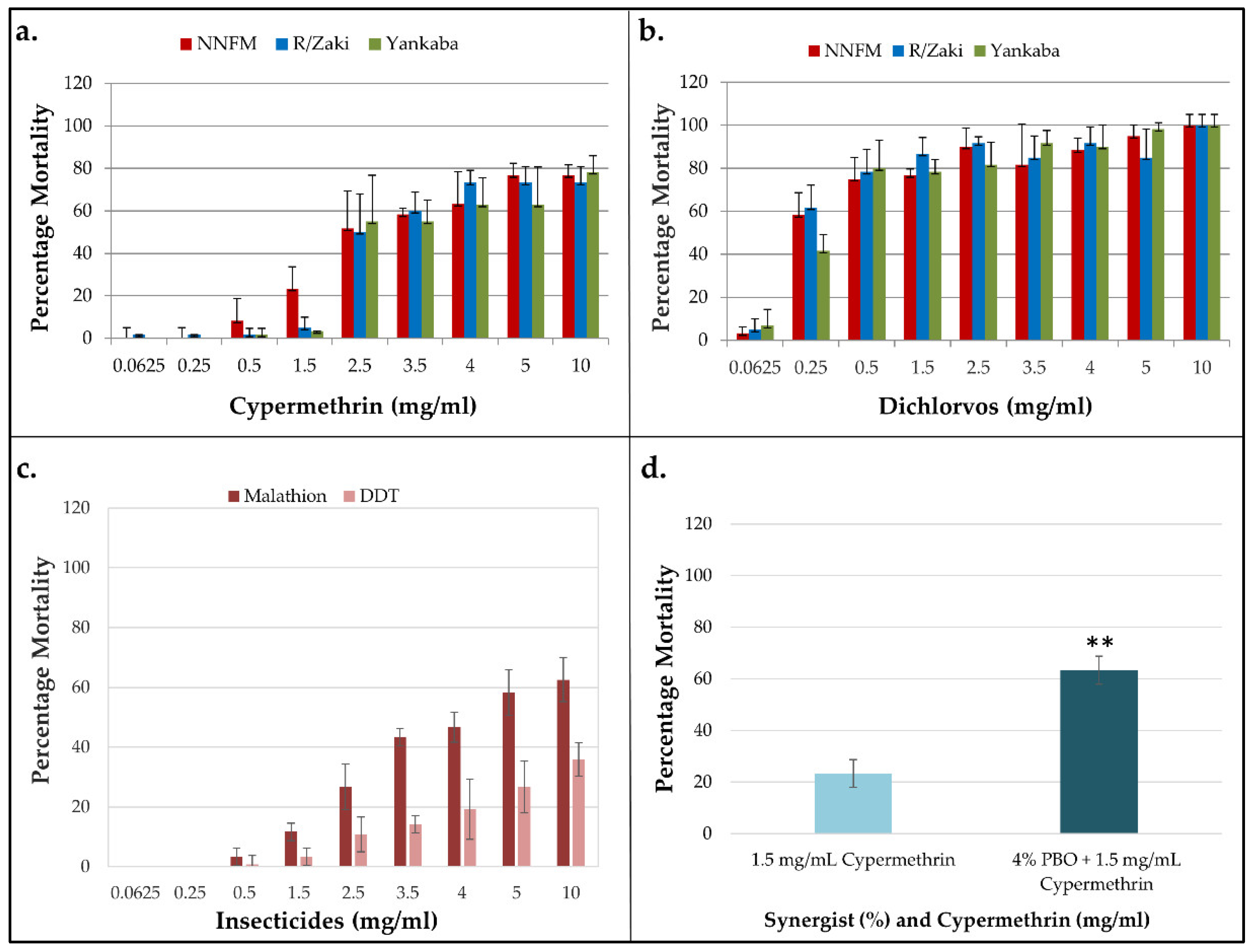
3.2. Insecticides Resistance Profile of the T. castaneum Populations
3.3. Assessment of the Role of Metabolic Resistance in Cypermethrin Resistance
3.4. Assessment of the Role of VGSC Target-Site Insensitivity Kdr Mutations in Cypermethrin Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- C. Lyddon, “Focus on Nigeria,” WORLD-GRAIN.com SOSLAND PUBLISHING COMPANY, 2022, 4, 88–100, Accessed: Jan. 22, 2022.
- J. Reidy, “Nigeria wheat initiative reaches first-year goal.,” SOSLAND PUBLISHING COMPANY 2022, www.world-grain.com. Available online: https://www.world-grain.com/articles/17449-nigeria-wheat-initiative-reaches-first-year-goal# (accessed on 9 December 2022).
- J. M. Turaki, B. M. Sastawa, B. G. J. Kabir, and N. E. S. Lale, “Susceptibility of flours derived from various cereal grains to infestation by the rust-red flour beetle (Tribolium castaneum Herbst) (Coleoptera: Tenebrionidae) in different seasons,” J. Plant Prot. Res. 2007, 47, 279–288. Available online: http://www.plantprotection.pl/Susceptibility-of-flours-derived-from-various-cereal-grains-to-infestation-by-the.91119.0.2.html.
- D. M. Mailafiya, Y. T. Maina, Y. M. Degri, and U. U. Gadzama, “Traders’ perception of food grain storage and pest management in Dalwa market, Borno State, Nigeria.” J. Agric Crop Res. 2014, 2, 62-70, ISSN: 2384-731X.
- R. Jagadeesan, P. J. Collins, G. J. Daglish, P. R. Ebert, and D. I. Schlipalius, “Phosphine resistance in the rust red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae): Inheritance, gene interactions and fitness costs,” PLoS ONE 2012, 7, 2012. [CrossRef]
- R. Ahmad, S. Hassan, S. Ahmad, S. Nighat, Y. K. Devi, K. Javeed, S. Usmani, M. J. Ansari, S. Erturk, M. Alkan and B. Hussain “Stored Grain Pests and Current Advances for Their Management,” in Postharvest Technology - Recent Advances, New Perspectives and Applications. IntechOpen, 2022. [CrossRef]
- 7. R. Baldwin and T. R. Fasulo, “Confused Flour Beetle, Tribolium confusum Jacquelin du Val and Red Flour Beetle, Tribolium castaneum (Herbst) (Insecta: Coleoptera: Tenebrionidae),” Edis, 1–5, 2020. [CrossRef]
- 8. E. M. Pires, E. Q. Souza, R. M. Nogueira, M. A. Soares, T. K. R. Dias, and M. A. Oliveira, “Damage caused by Tribolium castaneum (Coleoptera: Tenebrionidae) in Stored Brazil nut,” Sci. Electron. Arch. 2017, 10, 1–5. [CrossRef]
- 9. J. F. Campbell and C. Runnion, “Patch exploitation by female red flour beetles, Tribolium castaneum,” J. Insect Sci. 2003, 3, 20. https://pubmed.ncbi.nlm.nih.gov/15841236/. [CrossRef] [PubMed]
- M. I. Nafiu, N. Lawal, A. M. Aminu, A. S. Muohammed, R. I. Abdullahi, and T. A. Bello, “Productivity of Tribolium Species (Coleoptera; Tenebrionidae) within Flours Derived from Edible Tubers and Effects on Proximate Composition.” Int. J. Sci. Glob Sustain. 2020, 6, 12. https://fugus-ijsgs.com.ng/index.php/ijsgs/article/view/68.
- F. A. Ajayi and S. A. Rahman, “Susceptibility of some staple processed meals to red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae),” Pak. J. Biol. Sci. 2006, 9, 1744–1748, 2006. [CrossRef]
- C. N. Ehisianya, A. G. Stephen, and B. N. Onunka, “Development of Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) and Damage to Selected Flours in Storage,” Nig. Agric. J. 2022, 53, 193–198. https://www.ajol.info/index.php/naj/article/view/232673.
- S. Vojoudi, M. Saber, V. Mahdavi, H. Golshan, and Z. Abedi, “Efficacy of some Insecticides Against Red Flour Beetle, Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) Adults Exposed on Glass, Ceramic Tile, Plastic and Paper Disc Surfaces,” J. Lif Sci. 2012, 6, 405–410.
- H. Pieterse, G. G. M. Schulten, and W. Kuyken, “A study on insecticide resistance in Tribolium castaneum (Herbst) (Coleoptera, Tenebrionidae) in Malawi (Central Africa),” J. Stored Prod. Res. 1972, 8, 183–191. [CrossRef]
- Rauf and R. M. Wilkins, “Malathion-resistant Tribolium castaneum has enhanced response to oxidative stress, immunity, and fitness, Pest. Biochem. Physiol. 2022, 184. ISSN 0048-3575. [CrossRef]
- U. Shamjana and T. Grace, "Review of Insecticide Resistance and Its Underlying Mechanisms in Tribolium castaneum,” Insecticides - Impact and Benefits of Its Use for Humanity," Review of Insecticide Resistance and Its Underlying Mechanisms in Tribolium castaneum, 2022, IntechOpen. [CrossRef]
- E. Dyte and D. G. Blackman, “The spread of insecticide resistance in Tribolium castaneum (Herbst) (Coleoptera, Tenebrionidae),” J. Stored Prod. Res. 1970, 6, 255–261, 1970. [CrossRef]
- M. A. Attia, T. F. Wahba, N. Shaarawy, F. I. Moustafa, R. N. C. Guedes, and Y. Dewer, “Stored grain pest prevalence and insecticide resistance in Egyptian populations of the red flour beetle Tribolium castaneum (Herbst) and the rice weevil Sitophilus oryzae (L.),” J. Stored Prod. Res. 2020, 87, 101611. [CrossRef]
- F. K. Ayo, A. S. Hezekiah, and A. A. Temitope, “Comparative Pesticidal Activities of Essential Oils Extracted from Indigenous Plants Against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae),” Asian J. Sci. Res. 2019, 12, 502–507. [CrossRef]
- A. Ukeh and S. B. A. Umoetoka, “Repellent effects of five monoterpenoid odours against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) in Calabar, Nigeria,” Crop Prot. 2011, 30, 1351–1355. [CrossRef]
- A. Gbaye, E. A. Oyeniyi, E. I. Ogunleye, and O. V. Aloba, “The impact of cassava and wheat flour substrates on the biological parameters and insecticide response in Tribolium castaneum (Herbst),” Biocatal. Agric. Biotechnol. 2021, 38. [CrossRef]
- R. W. Beeman, S. Haas, and K. Friesen, “An Introduction to the Care and Handling of Tribolium castaneum,” USDA Tribolium stock maintenance, 2022. https://www.ars.usda.gov/plains-area/mhk/cgahr/spieru/docs/tribolium-stock-maintenance/.
- A. K. M. Dia, A. G. R. J. Sarr, A. Kafom, D. Ngom, T. Diome, C. Thiaw, S. Ndiaye and M. Sembene., “Morphological Identification of Trophic Tribolium Castaneum Populations Herbst (Coleoptera, Tenebrionidae) in West Africa.,” Int. J. Adv. Res. 2018, 6, 203–216. [CrossRef]
- R. Parthasarathy, A. Tan, H. Bai, and S. R. Palli, “Transcription factor broad suppresses precocious development of adult structures during larval–pupal metamorphosis in the red flour beetle, Tribolium castaneum R., 2008, 125, 299–313. [CrossRef]
- IRAC, “IRAC Test Methods Series. Test no. 006.,” Insecticides Resistance Action Committee Susceptibility., 2009, Version 3. https://irac-online.org/content/uploads/2009/09/Method_006_v3_june09.pdf (accessed on 2 February 2022).
- Bala, M. M. Mukhtar, H. K. Saka, N. Abdullahi, and S. S. Ibrahim, “Determination of insecticide susceptibility of field populations of tomato leaf miner (Tuta absoluta) in northern Nigeria,” Agriculture 2019, 9, 7. [CrossRef]
- Haddi, W. R. Valbon, L. O. Viteri Jumbo, L. O. de Oliveira, R. N. C. Guedes, and E. E. Oliveira, “Diversity and convergence of mechanisms involved in pyrethroid resistance in the stored grain weevils, Sitophilus spp.,” Sci. Rep. 2018, 8, 1–16. [CrossRef]
- R. A. Araújo, M. S. Williamson, C. Bass, L. M. Field, and I. R. Duce, “Pyrethroid resistance in Sitophilus zeamais is associated with a mutation (T929I) in the voltage-gated sodium channel,” Insect Mol. Biol. 2011, 20, 437–445. [CrossRef]
- J. Livak, “Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis,” Genetics, 1984, 107, 611–634. [CrossRef]
- T. Zhang, Y. Wang, W. Guo, D. Luo, Y. Wu, Z. Kučerová, V. Stejskal, G. Opit, Y. Cao, F. Li and Z. Li “DNA barcoding, species-specific PCR and real-Time PCR techniques for the identification of six Tribolium pests of stored products,” Sci. Rep., 2016, 6, 1–11. [CrossRef]
- W. S. Abbott, “A method of computing the effectiveness of an insecticide,” J. Econ. Entomol., 1925, 18, 265–267. [CrossRef]
- R. J. Hodges, J. C. Buzby, and B. Bennett, “Postharvest losses and waste in developed and less developed countries: Opportunities to improve resource use,” J. Agric. Sci., 2011, 149, 37–45. [CrossRef]
- Q. Ming, A. Wang, and C. Cheng, “Molecular identification of Tribolium castaneum and T. confusum (Coleoptera: Tenebrionidae) using PCR-RFLP analysis,” J. Genet., 2014, 93, e17–e21. https://pubmed.ncbi.nlm.nih.gov/24823304/.
- Y. Kayode, C. O. Adedire, and R. O. Akinkurolere, “Influence of four cereal flours on the growth of Tribolium castaneum Herbst (Coleoptera: Tenebrionidae),” Ife J. Sci. 2014, 16, 505–516.
- Rossi, S. Cosimi, and A. Loni, “Insecticide resistance in Italian populations of Tribolium flour beetles,” Bull. Insectolog. 2010, 63, 251–258, ISSN 1721-8861.
- Abo and E. Ja, “An evaluation of infestation of insect pests of flours in Benin City, Edo State, Nigeria,” J. Appl. Sci. Environ. Manag. 2014, 18, 487–494. Available online: https://www.ajol.info/index.php/jasem/article/view/109917.
- J. A. McFarlane, “Guidelines for pest management to reduce stored food losses caused by insects and mites.,” Greenwich Academic Literature Archive (GALA)., 1989, no. 22, p. 62. ODNRI Bulletin No. 22. Available online: https://gala.gre.ac.uk/id/eprint/10727/1/Doc-0115.pdf (accessed on 12 December 2022).
- S. Abouelkassem, A. A. Salem, and A. R. B. Arab, “Toxicity and development of resistance in Tribolium castaneum and Sitophilus oryzae oryzae to certain selected insecticides,” Egypt. J. Plant Prot. Res. Inst. 2018, 1, 188-198. http://www.ejppri.eg.net/pdf/v1n2/11.pdf.
- Naeem, S. I. Anjum, M. Ismail, J. Khan, S. Bibi., “Laboratory assessment of different botanical extracts and cypermethrin against insect pests,” J. Entomol. Zool. Stud. 2015, 3, 84–88. https://www.entomoljournal.com/archives/2015/vol3issue5/PartB/3-5-36.pdf.
- Khalequzzaman and M. Khanom, “Effects of cypermethrin alone and in combination with leaf and seed extracts of neem against adult Tribolium castaneum (Herbst),” Univ. J. Zool. Rajshahi Univ. 2006, 25, 45–49. [CrossRef]
- Andrić, P. Kljajić, I. Perić, and M. Pražić Golić, “Susceptibility of red flour beetle Tribolium castaneum (Herbst) populations from Serbia to contact insecticides,” 10th Int. Work. Conf. Stored Prod. Prot. 2010, 425, 869–873. [CrossRef]
- S. D. Reddy and C. Srivastava, “Persistent Toxicity of Malathion and Dichlorvos on Jute Surface Against Tribolium castaneum (Herbst),” Ann. Plant Prot. Sci. 2004, 12, 41–44. Available online: https://www.indianjournals.com/ijor.aspx?target=ijor:apps&volume=12&issue=1&article=011.
- E. A. Parkin, E. I. C. Scott, and R. Forster, "Increased resistance of stored-product insects to insecticides. The resistance of field strains of beetles," Pest Infest. Res. 1961, 34-35, 1962.
- B. R. Champ and M. J. Campbell-Brown, “Insecticide resistance in Australian Tribolium castaneum (Herbst) (Coleoptera, Tenebrionidae) - II. Malathion resistance in eastern Australia,” J. Stored Prod. Res. 1970, 6, 111–131. [CrossRef]
- G. GREENIN, “Malathion Resistance in the Red Flour Beetle,” Aust. J. Entomol. 1970, 9, 160–162. [CrossRef]
- C. E. Dyte and D. G. Blackman, “Selection of a DDT-resistant strain of Tribolium castaneum (Herbst) (Coleoptera, Tenebrionidae),” J. Stored Prod. Res. 1967, 2, 211–228. [CrossRef]
- R. Feyereisen, “Insect P450 inhibitors and insecticides: Challenges and opportunities,” Pest Manag. Sci. 2015, 71, 793–800. [CrossRef]
- S. S. Ibrahim et al., “Exploring the mechanisms of multiple insecticide resistance in a highly plasmodium-infected malaria vector Anopheles funestus sensu stricto from Sahel of northern Nigeria,” Genes 2020, 11, 454. [CrossRef]
- M. Mukhtar and S. S. Ibrahim, “Temporal Evaluation of Insecticide Resistance in Populations of the Major Arboviral Vector Aedes Aegypti from Northern Nigeria,” Insects 2022, 13, 187. [CrossRef]
- F. Zhu, R. Parthasarathy, H. Bai, K. Woithe, M. Kaussmann, R. Nauen, D. A. Harrison, and S. R. Palli., “A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum,” Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [CrossRef]
- C. T. Zimmer and R. Nauen, “Cytochrome P450 mediated pyrethroid resistance in European populations of Meligethes aeneus (Coleoptera: Nitidulidae),” Pestic. Biochem. Physiol. 2011, 100, 264–272. [CrossRef]
- J. Rösner, J. Tietmeyer, and H. Merzendorfer, “Functional analysis of ABCG and ABCH transporters from the red flour beetle, Tribolium castaneum,” Pest Manag. Sci. 2021, 77, 2955–2963. [CrossRef]
- M. Abd El Halim, B. M. H. Alshukri, M. S. Ahmad, E. Y. T. Nakasu, M. H. Awwad, E. M. Salama, A. M. R. Gatehouse and M. G. Edwards “RNAi-mediated knockdown of the voltage gated sodium ion channel TcNav causes mortality in Tribolium castaneum,” Sci. Rep. 2016, 6, 29301. [CrossRef]
- Rösner, B. Wellmeyer, and H. Merzendorfer, “Tribolium castaneum: A Model for Investigating the Mode of Action of Insecticides and Mechanisms of Resistance,” Curr. Pharm. Des. 2020, 26, no. 29, 3554–3568. [CrossRef]
- Dong, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov B.S., "Molecular biology of insect sodium channels and pyrethroid resistance." Insect Biochem Mol Biol. 2014, 50, 1-17. [CrossRef]
| Population | Insecticide | n | LC50 (mg/ml) (95% CI) |
|---|---|---|---|
| NNFM | Cypermethrin | 720 | 4.35 (3.68-5.01) |
| DDT | 720 | 15.32 (5.09-20.33) | |
| Dichlorvos | 720 | 0.28 (0.07-0.63) | |
| Malathion | 720 | 3.71 (1.72-5.92) | |
| R/Zaki | Cypermethrin | 720 | 4.45 (3.83-5.08) |
| Dichlorvos | 720 | 0.17 (0.03-0.46) | |
| Yankaba | Cypermethrin | 720 | 5.46 (4.64-6.27) |
| Dichlorvos | 720 | 0.35 (0.06-0.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





