1. Introduction
Soil fauna play a key role in soil C storage capacity, nutrient cycling and hydrology, that in turn affect soil quality [
1,
2,
3,
4]. However, the importance of soil organisms, including earthworms, in the delivery of ecosystem services is often overlooked, but it should be considered in future land management strategies, as healthy soil is an important resource to be protected [
5].
The importance of earthworms in agriculture has been studied since Darwin [
6]. In agricultural soils, earthworms contribute to soil health, quality and fertility, by increasing soil water and nutrient contents, soil macroporosity and aeration [
1,
7,
8,
9,
10,
11]. This occurs because soil fauna, and in particular earthworms, impact on soil physical, chemical and microbiological properties [
9,
10,
11]. Medina-Sauza et al. [
12] concluded that the positive effects of earthworms on soil fertility (in terms of nutrient availability, plant-growth promotion by signal molecules, soil water and C content) are mainly mediated via interactions with the microbial communities. As an example of earthworms-microorganisms’ feedbacks, the mucus secretion in the earthworm guts and deposited in their biogenic casts enhance the metabolism of plant-growth promoting soil microorganisms and soil biocontrol microbial agents [
13,
14]. In addition, the presence of earthworms is associated with increased C and N soil contents and a higher diversity of niches for microorganisms due to bioturbation [
4,
15]. Furthermore, earthworms also significantly increase soil microbial biomass and microbial respiration, being the main agents responsible for microbiological soil fertility [
1,
7,
11].
It is known that plant growth and development is affected by soil biota [
16]. At the same time, plants can influence soil biota, including earthworms, which in turn, speeds up the decomposition of plant litter [
17,
18,
19]. The study of microbial communities has increased dramatically thanks to the use of 16S rRNA metabarcoding, which allows a rapid screening of microbial diversity [
4,
20]. Standardized protocols to measure litter decomposition include the Tea Bag Index (TBI) method, which provides an easy way to measure the decay of plant material by using two standard types of tea bags (green and red tea) [
21]. The rates of organic matter decomposition measured with the TBI method have been found to be significantly related to microhabitat conditions, microbial diversity and types of agricultural practices adopted [
22,
23].
In this study, we designed a mesocosm experiment to test if and to what extent the presence of earthworms (
Eisenia fetida) affected a) soil bacterial community composition, b) litter decomposition, and c) plant growth (
Brassica oleracea, broccoli;
Vicia faba, faba bean). The epigeic earthworm
E. fetida was chosen for its proven role in soil quality improvement, as it is also present in compost, and for its plant growth-promoting effects [
3,
24,
25]. The two plant species were selected based on their opposite root architecture and morphology (thin, deep and dense taproot in broccoli; fibrous, shallow and diffuse root system in faba bean) [
26], that could be able to support different groups of soil microorganisms.
2. Results
2.1. Earthworm Abundance, Soil Abiotic Properties and Decomposition Rates, and Plant Growth
The scanned images acquired throughout the experiment allowed us to check the survival, abundance and activity of earthworms, confirming that they did not suffer in the pots (
Supplementary Figure S1). The number and total weight of earthworms doubled in
BRearth and
FBearth from the beginning to the end of the experiment, with the highest final values (107 specimens and 32.94 g total weight) observed in the
FBearth treatment (
Table 1).
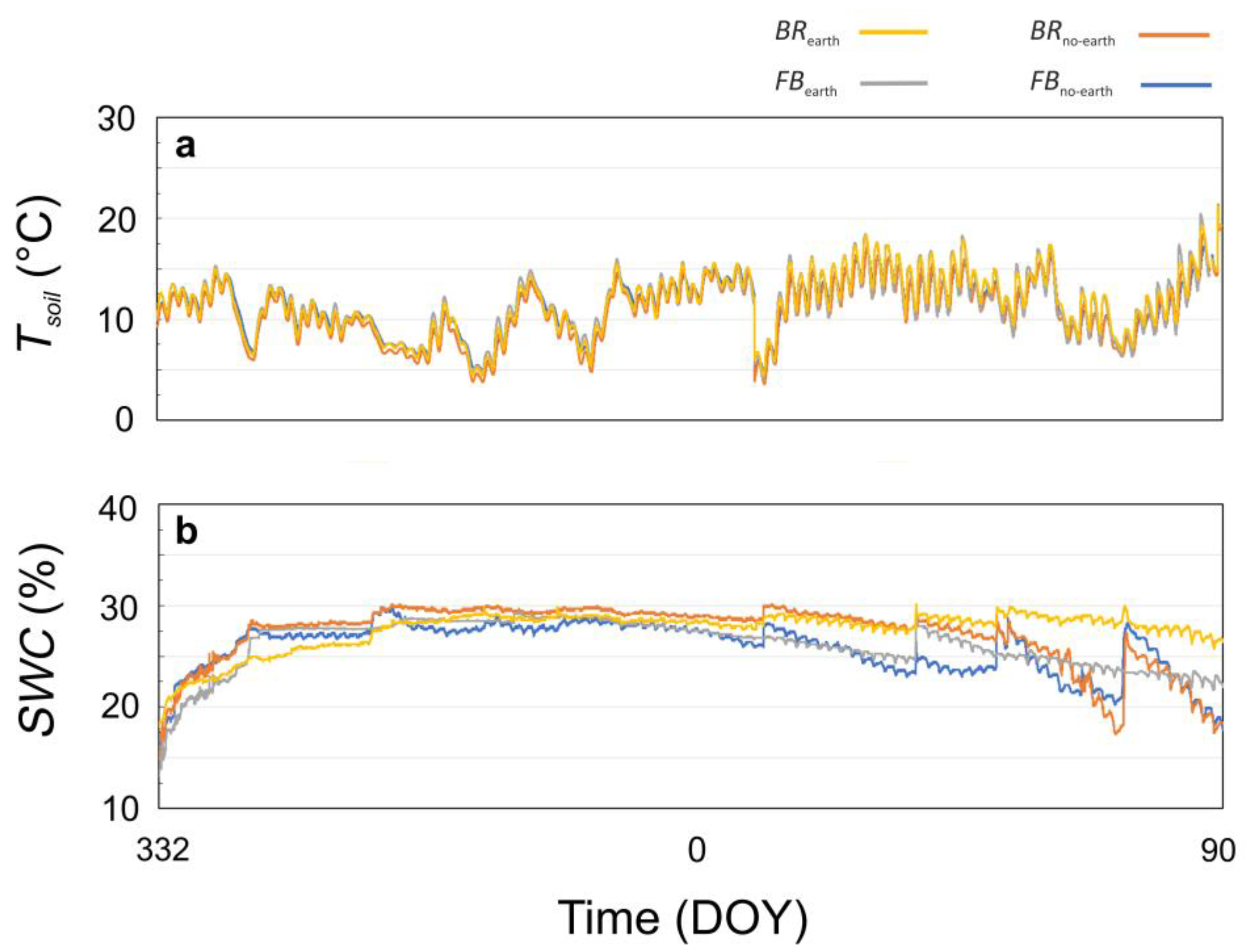
The values of
Tsoil did not significantly change among the treatments (
Figure 1a). Within each treatment, a certain degree of variation in
SWC was observed after a rainfall event, more pronounced in the second half of the trial, when plants were bigger, and in the treatments without earthworms (
Figure 1b). The treatments with earthworms, especially
BRearth, maintained more stable
SWC values throughout the experiment and a higher mean
SWC in the second half of the experiment compared to the treatments without earthworms (+7% in
BRearth and +5% in
FBearth) (
Figure 1b).
The presence of earthworms was generally associated with changes in the soil and litter physicochemical parameters, although the differences between treatments were not always statistically significant (
Figure 2,
Supplementary Table S2). Overall,
SOC,
STN and C/N decreased compared to the initial conditions (
Supplementary Table S1). However, the presence of earthworms significantly reduced the organic C content in the soil, litter bags and green tea bags, but not for red tea bags (
Figure 2a–d), more noticeable in the case of the broccoli treatments. The total N in the soil and green tea bags decreased with earthworms (significantly only in
FBearth) (
Figure 2e–g). Conversely, total N in the olive litter significantly increased for both plant species (
Figure 2f), while the total N in the red tea bags remained stable (
Figure 2h). The C/N ratios mirrored the observed differences in C and N contents, with lower values in the presence of earthworms (
Figure 2i–l), especially for
BRearth, with the exceptions of soil C/N in faba bean (
Figure 2i) and red tea C/N in broccoli (
Figure 2l).
Soil pH values were very similar in all treatments at the beginning of the experiment (on average 7.4) (
Supplementary Table S1), but significantly increased by the end of the trial in the pots containing earthworms (7.67 in
BRearth and 7.63 in
FBearth), in respect to pots without earthworms (7.17 in
BRno-earth and 7.15 in
FBno-earth) (
Figure 2m). The values of soil pH,
SOC,
STN,
TOCgreen,
TTNgreen,
TOCred,
TTNgreen,
LOC and
LTN measured at the beginning for the experiment are shown in
Supplementary Table S1.
Earthworms increased the
dlitter and
kTBI decomposition rates (Figures 2n–o), but decreased
STBI (
Figure 2p). More specifically,
dlitter was enhanced in the treatments with earthworms by about 53% in
BRearth and 41% in
FBearth, compared to the respective treatments without earthworms (
Figure 2n).
Significant differences in shoot growth due to earthworms were found only in faba bean (
Table 2). Regarding root traits, root maximum length and root fresh weight were influenced by the presence of earthworms both in broccoli and faba bean (
Table 2).
2.2. 16S rRNA Metabarcoding Analysis
2.2.1. α- and β-Diversity
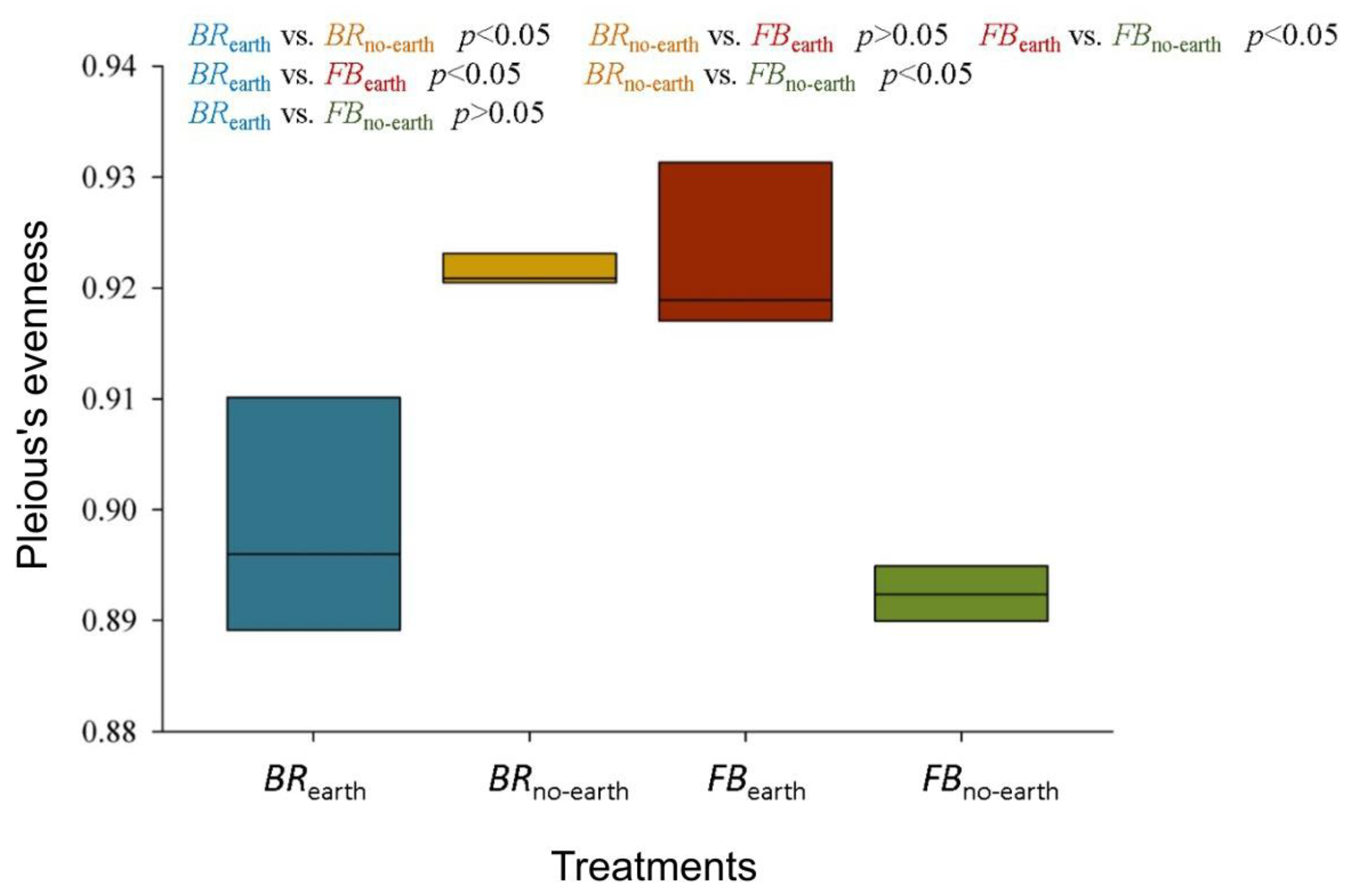
The
α-diversity analysis revealed a significant difference between treatments in terms of Pielou’s evenness index (
Figure 3). In addition, the bacterial communities in
BRearth were clearly distinct from those in
BRno-earth and
FBearth at
p < 0.05 (
Figure 3). Similarly, the Kruskal-Wallis test revealed a significant difference (p-value = 0.036) between
BRno-earth and
FBearth, as well as between
FBno-earth and
FBearth (
p < 0.05).
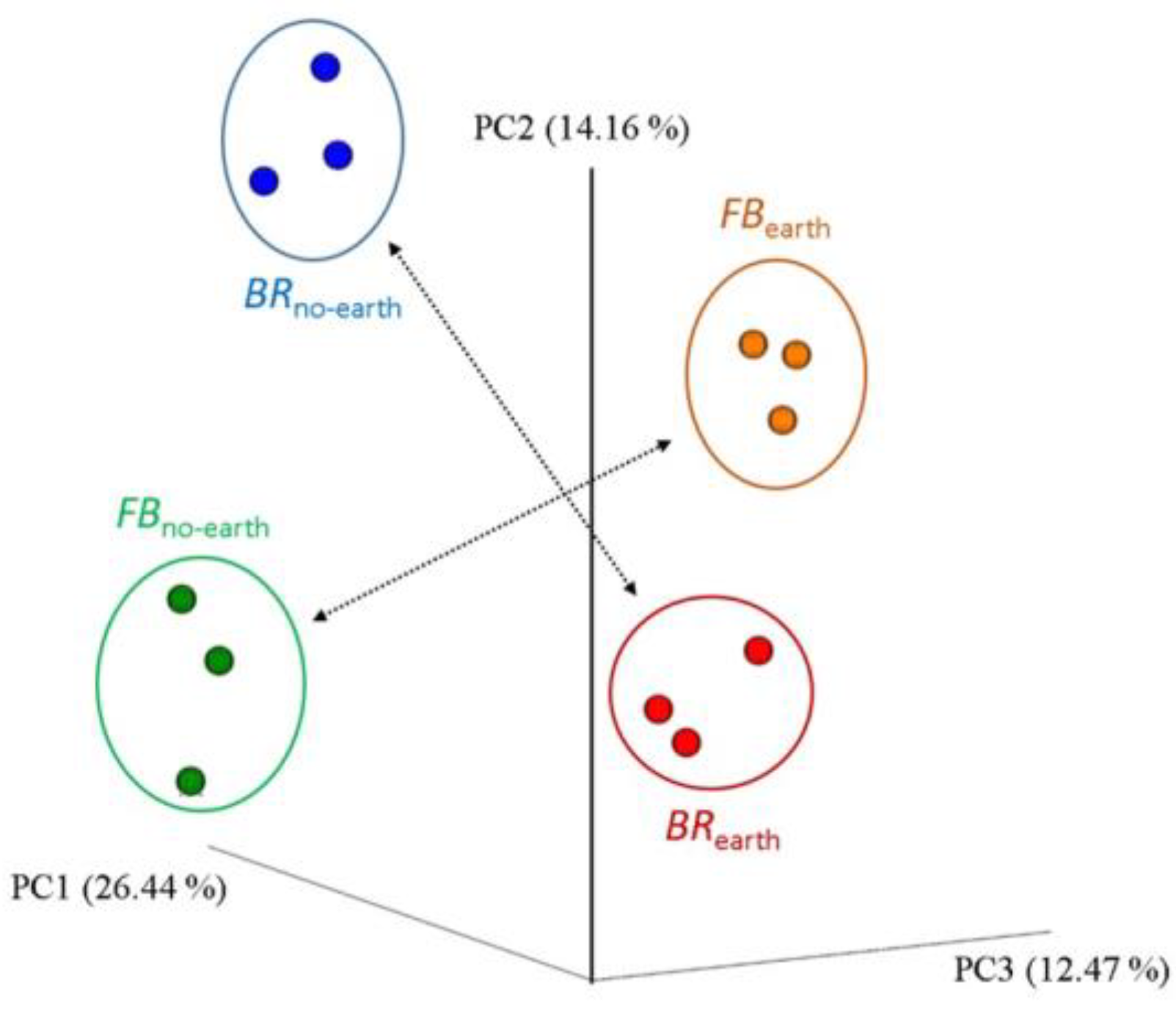
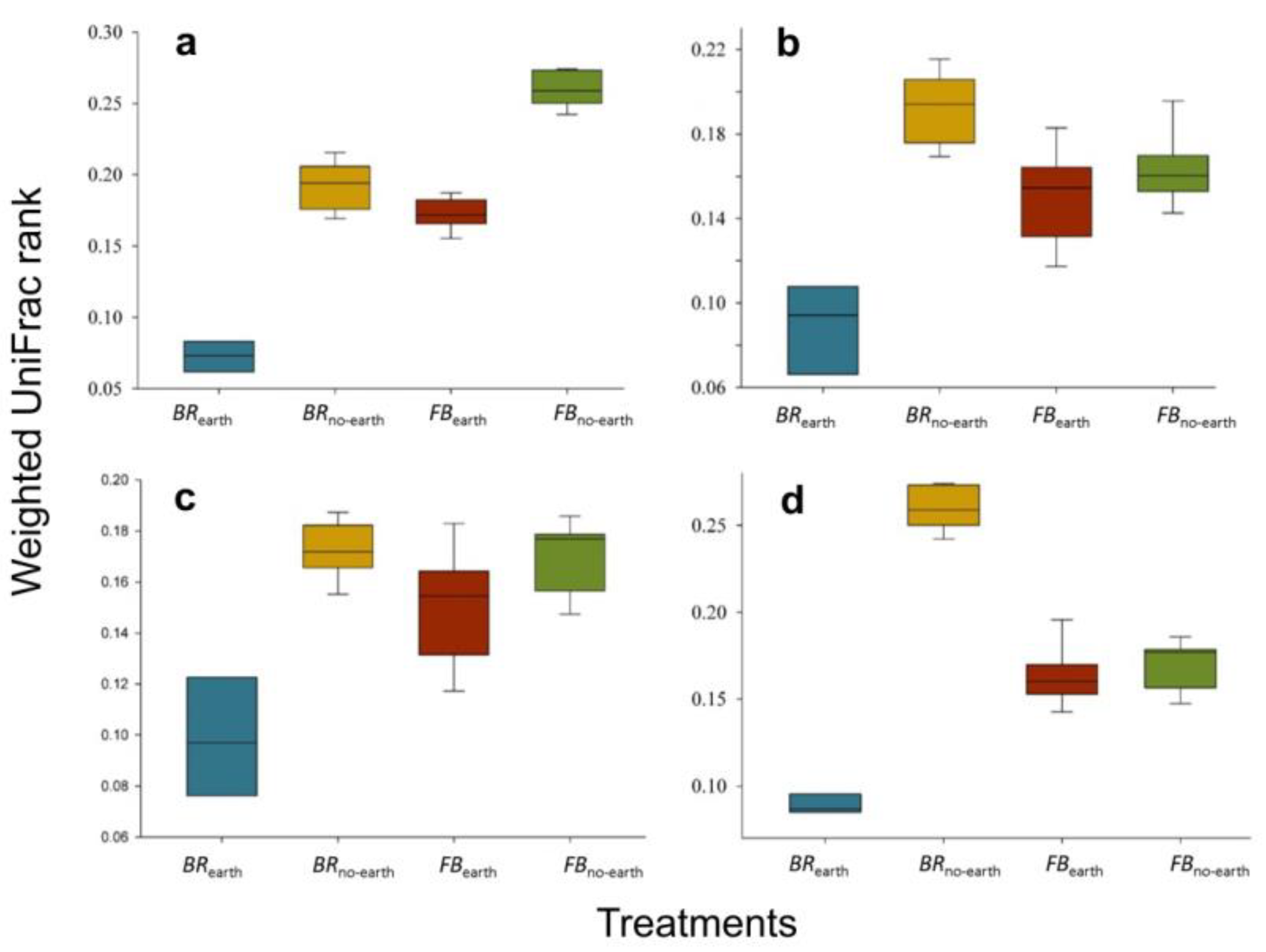
The extent of differences and similarities among bacterial communities was also explored using
β-diversity analysis. The principal coordinate analysis (PCoA) plot allows to visualize the existence of differences in
β-diversity between the bacterial communities in the different treatments based on Weighted Unifrac distance (phylogenetic method) (
Figure 4).
Significance was tested using ANOSIM with 999 permutations (
p value = 0.001). In addition, the treatments differed significantly, based on the analysis of similarities (
R value = 0.99,
p < 0.001) through weighted UniFrac distance (
Figure 5).
2.2.2. Bacterial Community Composition
As shown in
Table 3, a total of 11 Bacteria phyla (aggregate relative abundance > 1%) were identified in soil samples. Among them, Proteobacteria, Bacteroidota, Chloroflexi, Myxococcota, and Verrucomicrobia had a higher relative abundance in the
BRearth than in other sample types, while Patescibacteria, Firmicutes, Acidobacteria, and Gemmatimonadetes in
FBno-earth, and Actinobacteriota in
BRno-earth had a higher proportion. The results show that most phyla were influenced by the presence of earthworms and the type of cultivated plant. In addition,
Table 3 illustrates the results of Tukey's HSD test, in which the presence of earthworms significantly increased the abundance of Proteobacteria, Bacteroidota, Myxococcota, and Verrucomicrobia in the soil samples from
BRearth (+15.3, +15.6, +66.9, and +94.6%, respectively) and
FBearth (+14.8, +13.4, +31.9, and +6.6%, respectively), compared to their abundances in the same treatments without earthworms. In contrast, the abundances of Actinobacteriota, Firmicutes, Patescibacteria, Acidobacteria, and Gemmatimonadetes significantly decreased in
BRearth (–61.2, –102.1, –14.8, –23.4, and –67.4%, respectively) and
FBearth (–12.8, –48.7, –62.4, –24.5, and –20.6%, respectively).
Furthermore, some Bacteria families became relatively more abundant in soils with earthworm activity when compared to those without earthworms, such as BIrii41 (+94.6 and +135.7%), Devosiaceae (+59.4 and +25.4%), Pirellulaceae (+16.9 and +20.8%), Microscillaceae (+41.7 and +18.4%), and Microbacteriaceae (+80.7 and +135.1%) in broccoli and faba bean, respectively (
Table 3;
Supplementary Figure S3). On the other hand, the activity of earthworms significantly reduced the abundance of some bacterial families, such as Bacillaceae, Streptomycetaceae, Saprospiraceae, and Streptosporangiacea, which were 1.7, 8.4, 3.5, and 8.2 times lower in
BRearth compared to
BRno-earth, and 1.1, 0.9, 3.4, and 2.2 times lower in
FBearth compared to
FBno-earth, respectively (
Table 3;
Supplementary Figure S3). At the genus level, the most representative effect of earthworm activity was an increase in the abundance of many genera, the most important of which were
BIrii41 (+95.6 and +136.9%),
Devosia (+65.7 and +59.6%),
Flavobacterium (+431.1 and +961.5%), and
Ohtaekwangia (+330.6 and 1194.2%) in
BRearth and
FBearth, respectively (
Supplementary Figure S4). Conversely, earthworm activity reduced the abundance of some other genera, such as
Bacillus,
Nonomuraea, and
Streptomyces, by 170.7, 947.4, and 740.9% in
BRearth, and 102.8, 112.9, and 88.9% in
FBearth, respectively (
Supplementary Figure S4).
According to Ward's clustering method, the bacterial communities at the family and genus levels (relative abundance > 1%) clustered into several groups in which the
BRearth treatment was clearly separated from the rests, while
BRno-earth and
FBno-earth treatments were clustered into one group (
Supplementary Figures S3 and S4). On the basis of ANCOM method, of the 329 genera identified in the broccoli treatments, only
Opitutus (W=202),
Rubritalea (W=165),
Terrimonas (W=164), uncultured-
Verrucomicrobiaceae (W=156), and Bly10 (W=154) were present in
BRearth (
Supplementary Figure S5), whereas of the 285 genera found in faba bean treatments,
Paracoccus (W=160),
Kazania (W=134),
Caenimonas (W=121), and S-70 (W=118) were present only in
FBearth (
Supplementary Figure S6).
3.2.3. Quantitative PCR of the 16S rRNA Gene
Earthworm activity provided the larger set of significant 16S rRNA gene copies number in soil samples of both
BRearth (1.15 × 10
10 copies g
-1 of soil) and
FBearth (1.26 × 10
10 copies g
-1 of soil), which were significantly higher than
BRno-earth (+89%) and
FBno-earth (+223%). However, the type of cultivated plant had no significant effect on the ribosomal gene abundance in the examined soils (
Supplementary Figure S7).
Table 3.
Relative abundance of (a) bacterial phyla (relative abundance > 1%) and (b) families (relative abundance > 2%) in soils under different treatments. Means (± standard deviations) in each phylum or family followed by similar letters are not significantly different at 5% probability level (Tukey's HSD test).
Table 3.
Relative abundance of (a) bacterial phyla (relative abundance > 1%) and (b) families (relative abundance > 2%) in soils under different treatments. Means (± standard deviations) in each phylum or family followed by similar letters are not significantly different at 5% probability level (Tukey's HSD test).
| (a) |
Broccoli |
|
Faba bean |
| Phylum |
BRearth
|
BRno-earth
|
|
FBearth
|
FBno-earth
|
| Proteobacteria |
29.41 ± 1.96 a |
25.50 ± 1.14 bc |
|
26.07 ± 1.47 b |
22.71 ± 2.56 c |
| Bacteroidota |
21.57 ± 0.84 a |
18.66 ± 1.20 b |
|
21.26 ± 0.92 a |
18.74 ± 1.23 b |
| Actinobacteriota |
12.23 ± 0.97 c |
19.71 ± 1.55 a |
|
15.58 ± 1.57 bc |
17.58 ± 0.81 ab |
| Chloroflexi |
8.50 ± 0.58 a |
7.84 ± 0.60 a |
|
6.42 ± 0.11 b |
5.38 ± 0.19 b |
| Myxococcota |
8.61 ± 0.46 a |
5.16 ± 0.59 c |
|
6.40 ± 0.25 b |
4.85 ± 0.68 c |
| Planctomycetota |
6.35 ± 0.58 a |
6.32 ± 0. 63 a |
|
6.33 ± 0.45 a |
5.70 ± 0.41 a |
| Verrucomicrobia |
3.60 ± 0.26 a |
1.85 ± 0.16 c |
|
2.57 ± 0.32 b |
2.41 ± 0.32 bc |
| Firmicutes |
3.35 ± 0.34 b |
6.77 ± 0.75 a |
|
4.56 ± 0.41 ab |
6.78 ± 0.25 a |
| Patescibacteria |
2.76 ± 0.19 c |
3.17 ± 0.36 c |
|
5.96 ± 0.24 b |
9.68 ± 1.01 a |
| Acidobacteria |
1.11 ± 0.22 d |
1.37 ± 0.13 c |
|
1.63 ± 0.06 b |
2.03 ± 0.17 a |
| Gemmatimonadetes |
0.95 ± 0.07 c |
1.59 ± 0.22 ab |
|
1.41 ± 0.25 b |
1.70 ± 0.07 a |
| |
|
|
| (b) |
|
|
| Phylum |
Family |
BRearth
|
BRno-earth
|
|
FBearth
|
FBno-earth
|
| Bacteroidota |
Flavobacteriaceae |
7.11 ± 0.76 b |
7.97 ± 0.56 ab |
|
10.24 ± 1.11 a |
8.37 ± 0.59 ab |
| Myxococcota |
BIrii41 |
6.19 ± 0.66 a |
3.18 ± 0.24 c |
|
4.69 ± 0.54 b |
1.99 ± 0.39 c |
| Proteobacteria |
Devosiaceae |
5.34 ± 0.61 a |
3.35 ± 0.19 b |
|
2.76 ± 0.21 bc |
2.20 ± 0.14 c |
| Planctomycetota |
Pirellulaceae |
4.43 ± 0.26 a |
3.79 ± 0.45 b |
|
4.12 ± 0.56 ab |
3.41 ± 0.18 c |
| Bacteroidota |
Microscillaceae |
4.76 ± 0.35 a |
3.36 ± 0.26 b |
|
4.88 ± 0.24 a |
4.12 ± 0.44 ab |
| Chloroflexi |
SBR1031 |
2.77 ± 0.19 a |
1.53 ± 0.22 b |
|
1.13 ± 0.08 bc |
0.64 ± 0.07 c |
| Actinobacteriota |
Microbacteriaceae |
2.44 ± 0.31 a |
1.35 ± 0.21 b |
|
1.34 ± 0.08 b |
0.57 ± 0.08 c |
| Proteobacteria |
R7C24 |
2.13 ± 0.18 a |
0.72 ± 0.08 b |
|
0.82 ± 0.09 b |
0.33 ± 0.04 c |
| Firmicutes |
Bacillaceae |
1.29 ± 0.15 d |
3.48 ± 0.23 b |
|
2.15 ± 0.31 c |
4.50 ± 0.39 a |
| Actinobacteriota |
Streptomycetaceae |
0.22 ± 0.04 c |
1.84 ± 0.23 a |
|
1.08 ± 0.17 b |
2.04 ± 0.17 a |
| Bacteroidota |
Saprospiraceae |
0.30 ± 0.04 c |
1.04 ± 0.21 b |
|
0.70 ± 0.16 bc |
2.38 ± 0.31 a |
| Actinobacteriota |
Streptosporangiaceae |
0.25 ± 0.04 c |
2.04 ± 0.25 a |
|
1.12 ± 0.21 b |
2.41 ± 0.43 a |
3. Discussion
3.1. Earthworm Presence and Plant Species Affect Soil Bacterial Community via Different Soil Ecological Niches
Our results indicate that the observed changes in soil bacterial communities were associated with plant responses to earthworm activities (
Table 3;
Figure 3,
Figure 4 and
Figure 5). The outcome of the analyses of Bacteria
α- and
β-diversity for each plant species showed that bacterial community structure shifts were more pronounced for broccoli than for faba bean, especially in the presence of earthworms (
Figure 3 and
Figure 4). Yaghoubi Khanghahi et al. [
27] found that a different plant phenotypic response provides an altered habitat, probably via adjusted root architecture. In our study, these effects were more pronounced in soils with broccoli, with a thin, deep, and dense taproot, compared to faba bean, that has shallow, diffuse and longer roots [
4]. The different microbial changes between the two plant species (
Figure 3,
Figure 4 and
Figure 5) could also be due to different
SWC values (higher in broccoli due to the deeper root penetration into the soil) (
Figure 1) and soil C/N (lower in faba bean due to N fixation) (
Figure 2i). Recent studies by Gong et al. [
28] and Zhang et al. [
29] also support the view that soil microbial communities could be regulated by soil physicochemical properties, including nutrient factors (e.g., C/N ratio, total N, total P, etc.) and non-nutrient factors (e.g., vegetation cover, soil aggregate stability, pH, etc.). Besides the direct impacts of earthworms on soil bacterial community and diversity [
30], they can enhance soil bacterial activities measured as microbial respiration [
31] and increase soil microbial biomass and enzyme activity [
29].
The findings observed here (
Table 1; Figures 6 and 7;
Supplementary Figures S3 and S4) mirror those of previous studies that reported increasing abundance of some copiotrophic bacterial groups in response to the earthworm activities [
12,
28], such as Proteobacteria and Bacteroidota (with the dominant families of Flavobacteriaceae, Devosiaceae, Microscillaceae and R7C24) (
Table 1; Figures 6 and 7). In accordance with the hypothesis introduced by Männistö et al. [
32], the increase in the ratio between Proteobacteria and Acidobacteria in the earthworm-containing soils, compared to the treatments without earthworms (+42% in
BRearth and +43% in
FBearth), indicates better copiotrophic conditions in the former treatments, and confirms the ability of earthworms to alter the structure of the soil bacterial community under the two plant species studied here. Therefore, reductions in soil nutrient content and lower quality and quantity of organic C in those soils with earthworms led to the redundancy of ribosomal RNA gene copy numbers, as indicated by Männistö et al. [
32] and Khanghahi et al. [
33].
Conversely, the treatments lacking earthworms promote more oligotrophic Bacteria phyla such as Firmicutes, Patescibacteria, Acidobacteria and Gemmatimonadetes (
Table 1; Figures 6 and 7), and also a lower number of 16S rRNA gene copies (
Supplementary Figure S7). Moreover, the increase in Actinobacteria in the treatments without earthworms could be related to higher amounts of soil organic C and total N [
34]. This could explain their role as important decomposers of complex organic compounds with a great capability to degrade recalcitrant organic matter [
33]. In relation to this, He et al. [
35] reported a significant reduction in the biomass of soil Actinobacteria in the presence of earthworms. A possible explanation for this result may be the selective feeding and digestion of some specific taxa by earthworms, which led to reductions in the abundance of some bacterial groups [
35,
36].
In addition, plant-microbe interactions [47] and microbe-microbe symbiotic relationships occurring in the rhizosphere [
27] should also be taken into account together with their direct impact on soil bacterial communities. This was evident in the case of Flavobacteriaceae, the relatively most abundant family in our experiment, whose abundance changed in response to earthworm activity under broccoli and faba bean cultivation (
Table 1; Figures 6 and 7). In particular, the abundance of this bacterial family around the rhizoplane has been reported to be higher than in the rhizosphere and bulk soil and seems to be closely related to the type of cultivated plants [
38].
3.2. Earthworm-Driven Changes in Soil Physicochemical Parameters Resulted in Different Litter Decomposition Rates and Plant Growth
In our study, earthworms were responsible for most of the physicochemical changes observed in the soil (
Figure 1 and
Figure 2). The earthworm influence on soil macroporosity [
5,
8] was likely the main factor responsible for the observed higher
SWC content and stability (particularly evident during the second half of the experimental period) observed in both
BRearth and
FBearth, compared to the respective values found in
BRno-earth and
FBno-earth (
Figure 1b). In the case of soil pH (
Figure 2m), it is known that earthworm casts (small heaps of egested materials deposited on the soil surface and earthworm burrows) and mucus secretions can increase the soil pH [
25,
39], even if this action was likely attenuated by the acidifying actions of root exudates.
The excrements of earthworms are also rich in nutrients and microorganisms [
1,
7]. Therefore, they can be considered a natural fertilizer that contains five times more N, seven times more phosphorus and eleven times more calcium than the surrounding soil [
13,
14]. The observed values of
STN were higher in
BRno-earth and
FBno-earth, compared to the respective values measured in
BRearth and
FBearth (
Figure 2e), indicating that most of the readily available soil N was absorbed by roots and/or used for microbial growth. Interestingly,
STN was significantly higher in the faba bean treatments (
FBearth and
FBno-earth) compared to the broccoli ones (
BRearth and
BRno-earth) (
Figure 2e), possibly as a result of the N-fixing activity of root nodules in the legume. This also attenuated the differences in C/N ratio in the faba bean soils (
Figure 2i).
The earthworm-induced changes in soil physicochemical parameters were related to tea and olive litter decomposition rates (
Figure 2n–p). As hypothesized, earthworms accelerated the decay processes as confirmed by the increments in the decomposition rate constant (
kTBI), a decreased stabilization factor (
STBI) and greater litter mass losses (
dlitter) in the earthworm treatments (
Figure 2n–p). That earthworms enhance decomposition of different types of organic matter aligns well with the observed correlation between earthworm density and (tea) decomposition in the field [
18,
23]. In a greenhouse study however, no direct effect of earthworms on decomposition rates and stabilization was found [
40]. This may indicate that species identity and ecological grouping (anecic
Lumbricus terrestris in the previous study vs epigeic
Eisenia fetida in our study) or earthworm density might have also played a role (0.25 g L
‒1 vs 0.5 g L
‒1 in our study) (
Table 1).
The changes in physicochemical soil parameters and decomposition rates (
Figure 1 and
Figure 2) and the different structure of soil bacterial community due to earthworm presence (
Table 3;
Figure 3,
Figure 4 and
Figure 5) determined an accelerated plant growth, particularly evident in the root system (
Table 2).
4. Materials and Methods
4.1. Experimental Set-Up
The experimental area (Trani, BT, Puglia Region, Italy; 41°16'25"32 N, 16°24'58"32 E) is characterized by a semi-arid climate, with an average annual rainfall of 595 mm (1995-2021) and a mean annual temperature of 16.0 °C. The trial was carried out outdoors in the Autumn-Spring 2020-2021 (November-March). The experiment was performed on potted plants, using the same soil type and under the identical climatic conditions in a rainfed regime. This allowed eliminating any indirect effects due to initial leaf litter composition, soil type, or climate regime.
On 28 November 2020, three-week-old seedlings of broccoli (
Brassica oleracea L.) and fava bean (
Vicia faba L.), which were germinated in peat under controlled conditions (20 °C and 16:8 h light:dark photoperiod), were planted in a 30-L conical pots filled with local topsoil coming from an adjacent olive orchard (depth = 0-30 cm; sandy clay texture, with 45% sand, 14% silt and 41% clay) that was physicochemically characterized (electric conductivity = 0.159 mS cm
−1; total CaCO
3 = 4.2%, active CaCO
3 = 1.0%; assimilable phosphorus = 5 mg kg
−1; cation exchange capacity = 11.70 meq 100 g
−1, base saturation = 100%, Mg/K ratio = 1.78) (C/N and pH data in
Supplementary Table S1). Pots were incubated outside and exposed to the elements. Soil water content (
SWC) was not statistically different between pots at the beginning of the experiment (
Figure 1b), and therefore it was not necessary to adjust the soil moisture content. Eight pots per each plant species were established, each one with one plant seedling. Half of the pots (four of each plant species) received approximately 15 g (fresh weight) of mature, clitellated earthworms [
Eisenia fetida (Savigny, 1826)], that were previously counted, weighed and then gently mixed to the soil (earthworm treatments) (
Table 1); the remaining four pots for each plant species did not contain earthworms (control treatments). Earthworms were purchased from a local supplier (Fattoria Gallorosso Ssa; Matera, Italy). This resulted in four treatments replicated four times (broccoli with earthworms,
BRearth; faba bean with earthworms,
FBearth; broccoli without earthworms,
BRno-earth; and faba bean without earthworms,
FBno-earth). The experiment ended on 31 March 2021, giving a total time of 121 days.
4.2. Earthworm Abundance and Imaging
In
BRearth and
FBearth, earthworms were counted and weighed at the start (
Supplementary Figure S1) and at the end of the experiment (31 March). After emptying the pots on a plastic sheet, the earthworms were washed with tap water to remove any soil particles.
In two replicates of each of
BRearth and
FBearth, an image scanner (Canon CanoScan D646U; Canon Electronics Inc., Tokyo, Japan) without the upper cover lid, was placed in a sealed plastic bag and diagonally placed in the partially-filled pots, with the connecting USB cable emerging from it (
Supplementary Figure S2ab). Thereafter, the pots were completely filled with soil. This allowed the imaging of the soil inside the pot and of earthworm activity. Pictures were taken on six days (1 December, 3 January, 1 February, 28 February, 15 March, and 30 March) (
Supplementary Figure S1).
4.3. Soil Bacterial Community Structure
At the end of the trial (31 March), plants and soil were removed from the pots and placed on sterile plastic sheets. Soil was manually mixed using sterile gloves to make up a composite soil sample of about 1 kg. This sampling technique increased soil homogeneity t and allowed for the inclusion of the soil around mesh/litter bag, roots, in the bulk soil where earthworms were predominant, and sampled at different depths. After removing the visible root residues, the soil composite samples were stored in sterile plastic bags at 4 °C, and used for microbiological and chemical measurements within three days.
Soil DNA was extracted from 0.5 g of soil by using the soil DNA extraction kit (MP Biomedicals
TM FastDNA
TM Spin Kit). The DNA quality and concentration was checked using the NanoDrop spectrophotometer (ND-1000, EuroClone, Italy). All samples were diluted to a concentration of 20 ng mL
-1 and stored at −20 °C until the sequencing procedure. PCR of V3-V4 hypervariable regions of the 16S rRNA was performed by universal primers: 341F (5’-CCTACGGGNBGCASCAG-3’) and 805R (5’-GACTACNVGGGTATCTAATCC-3’). PCR and sequencing procedure was carried out by IGA Technology service (Udine, Italy,
https://igatechnology.com/) using an Illumina MiSeq next-generation sequencer (Illumina, San Diego, CA) with 300bp paired-end mode.
qPCR analysis was carried out to estimate bacterial rRNA gene copy numbers with the 515F/806R primer pairs. qPCR amplification in 20 μL volume contained 10 μL of iTaq Universal SYBR Green Supermix (2X; Bio-Rad, Hercules CA, USA), 0.4 μL of each primer (10 μM), 0.6 μL of BSA, 2 μL of template DNA, and 6.6 μL of nuclease-free water. The cycling conditions for the qPCR assay entailed enzyme activation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 57 °C for 60 s, and extension at 72 °C for 12 s. Amplification specificity was assessed by melting curves which were followed by ramping the temperature from 60 to 95 °C, with a reading every 0.5 °C. Standard curves were obtained using a series of 10-fold dilutions of PCR products amplified from the positive control samples which were extracted from the agarose gels using the NucleoSpin® Gel and PCR Clean-up (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) and quantified by Nanodrop spectrophotometer (ND-1000, EuroClone, Milan, Italy).
4.4. Litter Decomposition Rates
4.4.1. Tea Bag Index
On 28 November 2020, one green tea bag (
Camelia sinensis; n. EAN 87 10908 90359 5; Lipton) and one red tea (rooibos) bag (
Aspalanthus linearis; n. EAN 87 22700 18843 8; Lipton Unilever, Glascow, UK) were weighed and inserted 10 cm apart at 10 cm soil depth in each pot. The tea bags were retrieved after 90 days on 27 February 2021. Soil particles and roots were removed, and the tea bags oven-dried at 70 °C for 48 h and weighed. The Tea Bag Index (TBI) was calculated using the mass losses of red and green tea [
21]. The TBI describes the decay of labile material fractions expressed by the decomposition rate constant (
kTBI) that indicates the rate at which labile material fractions are decomposed and the stabilization factor (
STBI) that is a proxy for how much the labile fraction is not decomposed in the early stages of the decomposition process. Using the mass loss of the tea bags we calculated the Tea Bag Index (TBI) using a two phased decomposition model:
where
M(t) is the mass proportion of the substrate after incubation time
t in days,
a is the decomposed labile fraction of the litter,
1-a is the remaining fraction, and
k is the decomposition rate of the labile material fraction. After three months, green tea will lose very little mass with longer incubation and the remaining mass thus allows the decomposed fraction of green tea (
ag) to be calculated:
where
Mg(0) is the starting mass of green tea.
The fraction of the labile material that is not decomposed by microorganisms, but stabilized (
STBI), was then calculated using the hydrolysable fraction of green tea (
Hg):
Assuming that
STBI is equal for red and green tea, and using the hydrolysable fraction of red tea (
Hr), the decomposed fraction of red tea (
ar) was calculated, from which
kTBI was derived using Equation 1.
4.4.2. Litter Bags
Litter bags with a size of 20 × 15 cm were prepared using non-decomposable tulle fabric (TFT Spa; Segrate, Milan, Italy) and filled with chemically characterized leaf litter (organic carbon = 42.10 g kg
‒1; total nitrogen = 5.75 g kg
‒1) collected from an adjacent olive orchard, dried at 25 °C for 15 days and weighed separately. The bag mesh size of 1 mm allowed microorganisms and small mesofauna to enter the bags but excluded macrofauna [
2]. On 28 November 2020, the litter bags were buried at 10 cm depth in each pot and retrieved on 31 March 2021. Thereafter, the surrounding roots were cut off and the attached soil was carefully removed and finally, thel litter bags were oven-dried at 70 °C for 48 h and weighed. The difference between the initial and post-incubation total weights of the litter bags were used for calculating the percentage mass loss due to litter decomposition (
dlitter).
4.5. Physicochemical Analyses
Soil temperature (
Tsoil) and
SWC were monitored throughout the experiment at 10-cm depth and 10-min intervals in one replicate per treatment. Soil temperature was measured by DS18B20 digital sensors (Maxim Integrated, San Jose, USA) calibrated by analogical thermometers (Brannan, Cleator Moore, United Kingdom), whereas soil moisture was measured by capacitive sensors (Seeed Studio, Shenzhen, China) (
Supplementary Figure S2ce) and expressed as percentage of soil dry weight (drying at 105 °C for 24 h). All sensors were controlled by a board Arduino UNO with an integrated microcontroller ATMEGA328P (Arduino s.r.l., Monza, Italia), and data recorded by a DS3231 datalogger (Adafruit Industries, New York, USA) (
Supplementary Figure S2cd).
Composite soil samples were taken from each pot at the end of the experiment (31 March). The soil was dried at 105 °C for 24 h, placed in a desiccator until a constant weight was reached, and then sieved through a 2-mm stainless steel sieve. The organic carbon and total nitrogen content in the soil (
SOC and
STN, respectively), tea bags (
TOC and
TTN, respectively) and litter (
LOC and
LTN, respectively) were determined at the end of the experiment (27 February 2021 only for
TOC and
TTN, whereas 31 March for the other parameters). Organic carbon was determined by the Walkley and Black method by oxidation at 170 °C with potassium dichromate (K
2Cr
2O
7) in the presence of sulfuric acid (H
2SO
4), and the excess K
2Cr
2O
7 was measured by Möhr salt titration, while total N was measured by the Kjeldahl method [
41]. Soil pH was measured by a glass electrode (Basic 20
®; Crison Instruments SA, Barcelona, Spain) by suspending soil in distilled water (1:2.5 soil to liquid phase ratio). For all these parameters, C/N ratios were calculated dividing the values of organic carbon by total N. The values of
SOC,
STN, soil pH,
TOC,
TTN,
LOC and
LTN were also measured at the beginning of the experiment (28 November) on random soil, tea bag and litter samples.
4.6. Plant Growth Parameters
At the end of the experiment, plants were carefully removed from the pots after wetting the soil to avoid root damage. Shoots were separated from the roots by a scalpel. Shoot maximum height was measured using a ruler and then whole shoots (including stem, leaves and fruits) were weighted (fresh weight). Then, the roots were cleaned by washing off the excess of soil using tap water and slightly dried with an absorbent cloth. The root maximum length, which is an estimate of the rooting depth, was measured using a ruler and then root fresh weight was recorded.
4.7. Bioinformatics
For the 16S rRNA metabarcoding analysis, paired-end Illumina sequencing raw reads were imported into Microbial Ecology 2 software (QIIME2 2021.8 distribution,
https://qiime2.org/) [
42].
The total number of reads obtained from the sequencing run was 1,872,622. Forward and reverse reads (approximately 936,000 each) were quality filtered, denoised, paired-end reads merged using DADA2 pipeline [
43] which includes removal of chimeric reads. Further analyses were performed by Qiime2 at a sampling depth of 19,810 sequences per sample in order to normalize all samples to the size of the less abundant one, maintaining the richness of the dataset. A phylogenetic tree was generated by a phylogeny pipeline using the script “qiime diversity analysis align-to-tree-mafft-fasttree”. To compare bacterial community structure of treatments alpha diversity analyses were carried out on ASVs (amplicons sequence variants) data. We used the script “qiime diversity group significance” to test alpha diversity metrics (observed ASVs, Faith’s Phylogenetic Diversity, Shannon diversity index, Pielou’s evenness index) and to compare both species richness and evenness within samples. Beta diversity was analyzed by “beta-group-significance” scripts by Weighted UniFrac distance matrix, which incorporates phylogenetic distances between observed organisms, to compare diversity in the community composition between treatments. Naïve Bayesian classifier was used for taxonomic classification against the SILVA database (
https://www.arb-silva.de/) using the script “qiime feature-classifier classify-sklearn”. Data analysis bar charts (
Supplementary Figure S4) were created at phylum and class level taxonomic assignments for each replicate sample.
4.8. Statistical Analyses and Data Visualization
The impact of the earthworms, in interaction with the plant species, on the soil and litter physicochemical properties was assessed by means of two way ANOVA followed by Post-Hoc Tukey's Honest Significant Difference (HSD) tests, in the R statistical environment (
https://www.r-project.org/) to determine the effect of earthworm presence and plant species on the studied parameters.
The
α-diversity statistical analysis was conducted in Qiime2 using Kruskal-Wallis test (
Figure 3). The
β-diversity statistical analysis was conducted in Qiime2 using ANOSIM test with 999 permutations and visualized by PCoA plot (
Figure 4). Ward's clustering method, expressed by Euclidean distance, was used to compute the distance among bacterial community compositions in response to the presence of earthworms and species of cultivated plant. (
Figure 5). ANCOM (Analysis of Composition of Microbiomes) in Qiime2 [
44] was carried out at genus level using q2-composition pipeline after removing the zeros by q2-pseudocount. The volcano plots (
Supplementary Figure S5 and S6) represent the ANCOM visualization where x-axis is centered by log-ratio (clr) of F statistic and W value of y-axis represents the number of rejected null hypotheses (that is, the average abundance of a given species in a group is the same to that in the other group).
5. Conclusions
Our findings allow us to conclude that earthworms had a significant impact on plant growth, soil bacterial community structure, and on litter decomposition. From an agricultural point of view, the presence of earthworms allowed roots of both broccoli and faba bean to grow better in soils that accumulate high levels of nutrients. Therefore, earthworms can be seen as a potential nature-based solution to ensure the sustainable use and conservation of soils, including adaptation and resilience to climate change, and for the long-term biological sustainability of soil systems. In the future, a repetition of this experiment with agricultural earthworm species (e.g., L. terrestris, A. caliginosa, A. rosea), also including perennial crops, would greatly enhance the potential of this study for transferring the results to practitioners, farmers, SMEs, policy makers and related end users for designing sustainable land use systems in different soils and climates.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, A.S..; methodology, A.S., J.S. and C.C.; software, F.R. and D.C..; formal analysis, F.R. and M.J.I.B; investigation, A.S., M.Y.K. and M.C..; resources, A.S. and C.C.; data curation, A.S., M.Y.K., M.C. and J.S.; writing—original draft preparation, A.S., M.Y.K. and M.C.; writing—review and editing, A.S. and M.J.I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; Pulleman, M.; Sukkel, W.; van Groenigen, J.W.; Brussaard, L. Soil quality – A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Comparing the effects of soil fauna on litter decomposition and organic matter turnover in sustainably and conventionally managed olive orchards. Geoderma 2020, 372, 114393. [Google Scholar] [CrossRef]
- Žaltauskaitė, J.; Kniuipytė, I.; Praspaliauskas, M. Earthworm Eisenia fetida potential for sewage sludge amended soil valorization by heavy metal remediation and soil quality improvement. J. Hazard. Mater. 2022, 424, 127316. [Google Scholar] [CrossRef]
- Sofo, A.; Ricciuti, P.; Fausto, C.; Mininni, A.N.; Crecchio, C.; Scagliola, M.; Malerba, A.D.; Xiloyannis, C.; Dichio, B. The metabolic and genetic diversity of soil bacterial communities depends on the soil management system and C/N dynamics: The case of sustainable and conventional olive groves. Appl. Soil Ecol. 2019, 137, 21–28. [Google Scholar] [CrossRef]
- Ponge, J.F. The soil as an ecosystem. Biol. Fertil. Soils 2015, 51, 645–648. [Google Scholar] [CrossRef]
- Darwin, C.R. The Formation of Vegetable Mould, through the Action of Worms, with Observations on their Habits; John Murray: London, UK, 1881; http://darwin-online.org.uk/EditorialIntroductions/Freeman_VegetableMouldandWorms.html (accessed January 23, 2023). [Google Scholar]
- Seeber, J.; Seeber, G.U.H.; Langel, R.; Scheu, S.; Meyer, E. The effect of macro-invertebrates and plant litter of different quality on the release of N from litter to plant on alpine pastureland. Biol. Fertil. Soils 2008, 44, 783–790. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.K.; Schröder, B. Perspectives in modelling earthworm dynamics and their feedbacks with abiotic soil properties, Appl. Soil Ecol. 2012, 58, 29–36. [Google Scholar] [CrossRef]
- Briones, M.J.I. Soil fauna and soil functions: A jigsaw puzzle. Front. Environ. Sci. 2014, 2, 7. [Google Scholar] [CrossRef]
- Lavelle, P. Functional domains in soils. Ecol. Res. 2002, 17, 441–450. [Google Scholar] [CrossRef]
- Medina-Sauza, R.M.; Álvarez-Jiménez, M.; Delhal, A.; Reverchon, F.; Blouin, M.; Guerrero-Analco, J.A.; Cerdán, C.R.; Guevara, R.; Villain, L.; Barois, I. Earthworms building up soil microbiota, a review. Front. Environ. Sci. 2019, 7, 81. [Google Scholar] [CrossRef]
- Furlong, M.A.; Singleton, D.R.; Coleman, D.C.; Whitman, W.B. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 2002, 68, 1265–1279. [Google Scholar] [CrossRef]
- Bhadauria, T; Saxena, K.G. Role of earthworms in soil fertility maintenance through the production of biogenic structures. Appl. Environ. Soil Sci. 2010, 816073. [Google Scholar] [CrossRef]
- Fahey, T.J.; Yavitt, J.B.; Sherman, R.E.; Maerz, J.C.; Groffman, P.M.; Fisk, M.C.; Bohlen, P.J. Earthworm effects on the incorporation of litter C and N into soil organic matter in a sugar maple forest. Ecol. Appl. 2013, 23, 1185–1201. [Google Scholar] [CrossRef] [PubMed]
- De Deyn, G.B.; Raaijmakers, C.E.; Van Der Putten, W.H. Plant community development is affected by nutrients and soil biota. J. Ecol. 2004, 92, 824–834. [Google Scholar] [CrossRef]
- Chen, G.; Cao, Y.; Tang, Y.; Yang, X.; Liu, Y.; Huang, D.; Zhang, Y.; Li, C.; Wang, Q. Advanced near-infrared light for monitoring and modulating the spatiotemporal dynamics of cell functions in living systems. Adv. Sci. 2020, 7, 1903783. [Google Scholar] [CrossRef] [PubMed]
- Tresch, S.; Frey, D.; Le Bayon, R.C.; Zanetta, A.; Rasche, F.; Fliessbach, A.; Moretti, M. Litter decomposition driven by soil fauna, plant diversity and soil management in urban gardens. Sci. Total Environ. 2019, 658, 1614–1629. [Google Scholar] [CrossRef] [PubMed]
- Ayres, E.; Steltzer, H.; Berg, S.; Wall, D.H. Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. J. Ecol. 2009, 97, 901–912. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef] [PubMed]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Pioli, S.; Sarneel, J.; Thomas, H.J.D.; Domene, X.; Andrés, P.; Hefting, M.; Reitz, T.; Laudon, H.; Sandén, T.; Piscová, V.; Aurela, M.; Brusetti, L. Linking plant litter microbial diversity to microhabitat conditions, environmental gradients and litter mass loss: Insights from a European study using standard litter bags, Soil Biol. Biochem. 2020, 144, 107778. [Google Scholar] [CrossRef]
- Spiegel, H.; Mosleitner, T.; Sandén, T.; Zaller, J.G. Effects of two decades of organic and mineral fertilization of arable crops on earthworms and standardized litter decomposition. Bodenkultur 2018, 69, 17–28. [Google Scholar] [CrossRef]
- Garau, M.; Garau, G.; Sizmur, T.; Coole, S.; Castaldi, P.; Pinna, M.V. Biochar and Eisenia fetida (Savigny) promote sorghum growth and the immobilization of potentially toxic elements in contaminated soils. App. Soil Ecol. 2023, 182, 104697. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia fetida (Sav.). Bioresour. Technol. 2018, 250, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Elshafie, H.S.; Camele, I. Structural and functional organization of the root system: A comparative study on five plant species. Plants 2020, 9, 1338. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi Khanghahi, M.; Crecchio, C.; Verbruggen, E. Shifts in the rhizosphere and endosphere colonizing bacterial communities under drought and salinity stress as affected by a biofertilizer consortium. Microb. Ecol. 2021, 84, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Jiang, Y.; Zheng, Y.; Chen, X.; Li, H.; Hu, F.; Liu, M.; Scheu, S. Earthworms differentially modify the microbiome of arable soils varying in residue management. Soil Biol. Biochem. 2018, 121, 120–129. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, B.J.; Bi, Q.F.; Li, K.J.; Sun, C.L.; Lin, X.Y.; Zhu, Y.G. Variations of earthworm gut bacterial community composition and metabolic functions in coastal upland soil along a 700-year reclamation chronosequence. Sci. Total Environ. 2022, 804, 149994. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, S.; Wang, Z.; Jiang, Y.; Hu, Z.; Zheng, Y.; Chen, X.; Li, H.; Hu, F.; Liu, M.; Scheu, S. Earthworms modify soil bacterial and fungal communities through enhancing aggregation and buffering pH. Geoderma 2019, 347, 59–69. [Google Scholar] [CrossRef]
- Luo, S.; Ren, L.; Wu, W.; Chen, Y.; Li, G.; Zhang, W.; Wei, T.; Liang, Y.Q.; Zhang, D.; Wang, X.; Zhen, Z.; Lin, Z. Impacts of earthworm casts on atrazine catabolism and bacterial community structure in laterite soil. J. Hazard. Mater. 2022, 425, 127778. [Google Scholar] [CrossRef]
- Männistö, M.; Ganzert, L.; Tiirola, M.; Häggblom, M.M.; Stark, S. Do shifts in life strategies explain microbial community responses to increasing nitrogen in tundra soil? Soil Biol. Biochem. 2016, 96, 216–228. [Google Scholar] [CrossRef]
- Khanghahi, M.Y.; Murgese, P.; Strafella, S.; Crecchio, C. Soil biological fertility and bacterial community response to land use intensity: a case study in the Mediterranean area. Diversity 2019, 11, 211. [Google Scholar] [CrossRef]
- Dong, W.; Liu, E.; Yan, C.; Tian, J.; Zhang, H.; Zhang, Y. Impact of no tillage vs. conventional tillage on the soil bacterial community structure in a winter wheat cropping succession in northern China. Eur. J. Soil Biol. 2017, 80, 35–42. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Liu, T.; Yang, X.; Cao, J.; Tao, L.; Wang, X.; Liu, Z.; Yao, Q.; Li, Y.; Zou, X.; Shao, Y.; Li, J.; Zhang, W.; Fu, S. Earthworms negate the adverse effect of arbuscular mycorrhizae on living bacterial biomass and bacterial necromass accumulation in a subtropical soil. Soil Biol. Biochem. 2020, 151, 108052. [Google Scholar] [CrossRef]
- Shan, J.; Liu, J.; Wang, Y.; Yan, X.; Guo, H.; Li, X.; Ji, R. Digestion and residue stabilization of bacterial and fungal cells, protein, peptidoglycan, and chitin by the geophagous earthworm Metaphire guillelmi. Soil Biol. Biochem. 2013, 64, 9–17. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef]
- Kraut-Cohen, J.; Shapiro, O.H.; Dror, B.; Cytryn, E. Pectin induced colony expansion of soil-derived flavobacterium strains. Front. Microbiol. 2021, 12, 544. [Google Scholar] [CrossRef]
- Wen, B.; Hu, X.Y.; Liu, Y.; Wang, W.S.; Feng, M.H.; Shan, X.Q. The role of earthworms (Eisenia fetida) in influencing bioavailability of heavy metals in soils. Biol. Fertil. Soils. 2004, 40, 181–187. [Google Scholar] [CrossRef]
- Van Hoesel, W.; Tiefenbacher, A.; König, N.; Dorn, V.M.; Hagenguth, J.F.; Prah, U.; Widhalm, T.; Wiklicky, V.; Koller, R.; Bonkowski, M.; Lagerlöf, J.; Ratzenböck, A.; Zaller, J.G. Single and combined effects of pesticide seed dressings and herbicides on earthworms, soil microorganisms, and litter decomposition. Front. Plant Sci. 2017, 8, 215. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin, Germany, 2006. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; Huttley, G.A.; Kelley, S.T.; Knights, D.; Koenig, J.E.; Ley, R.E.; Lozupone, C.A.; McDonald, D.; Muegge, B.D.; Pirrung, M.; Reeder, J.; Sevinsky, J.R.; Turnbaugh, P.J.; Walters, W.A.; Widmann, J.; Yatsunenko, T.; Zaneveld, J.; Knight, R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
(a) Soil water content (SWC) and (b) soil temperature (Tsoil) measured throughout the experimental period in soils under different treatments, consisting of broccoli with earthworms (BRearth: yellow), broccoli without earthworms (BRno-earth: red), faba bean with earthworms (FBearth: grey), and faba bean without earthworms (FBno-earth: blue). DOY: day of year.
Figure 1.
(a) Soil water content (SWC) and (b) soil temperature (Tsoil) measured throughout the experimental period in soils under different treatments, consisting of broccoli with earthworms (BRearth: yellow), broccoli without earthworms (BRno-earth: red), faba bean with earthworms (FBearth: grey), and faba bean without earthworms (FBno-earth: blue). DOY: day of year.
Figure 2.
Boxplots of soil physico-chemical parameters in soil and litter under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). (a) Soil organic carbon (SOC); (b) litter organic carbon (LOC); (c) organic carbon in green tea bags (TOCgreen); (d) organic carbon in red tea bags (TOCred); (e) soil total nitrogen (STN); (f) litter total nitrogen (LTN); (g) total nitrogen in green tea bags (TTNgreen); (h) total nitrogen in red tea bags (TTNred); (i) soil carbon to nitrogen ratio (Soil C/N); (j) litter carbon to nitrogen ratio (Litter C/N); (k) carbon to nitrogen ratio in green tea bags (Green tea C/N); (l) carbon to nitrogen ratio in red tea bags (Red tea C/N); m) soil pH (pH); (n) percentage of litter decomposed (dlitter); (o) decomposition constant of tea bags (kTBI); (p) stabilization factor of tea bags (STBI). Different letters indicate significant differences after two-way ANOVA followed by Tukey's HSD test.
Figure 2.
Boxplots of soil physico-chemical parameters in soil and litter under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). (a) Soil organic carbon (SOC); (b) litter organic carbon (LOC); (c) organic carbon in green tea bags (TOCgreen); (d) organic carbon in red tea bags (TOCred); (e) soil total nitrogen (STN); (f) litter total nitrogen (LTN); (g) total nitrogen in green tea bags (TTNgreen); (h) total nitrogen in red tea bags (TTNred); (i) soil carbon to nitrogen ratio (Soil C/N); (j) litter carbon to nitrogen ratio (Litter C/N); (k) carbon to nitrogen ratio in green tea bags (Green tea C/N); (l) carbon to nitrogen ratio in red tea bags (Red tea C/N); m) soil pH (pH); (n) percentage of litter decomposed (dlitter); (o) decomposition constant of tea bags (kTBI); (p) stabilization factor of tea bags (STBI). Different letters indicate significant differences after two-way ANOVA followed by Tukey's HSD test.
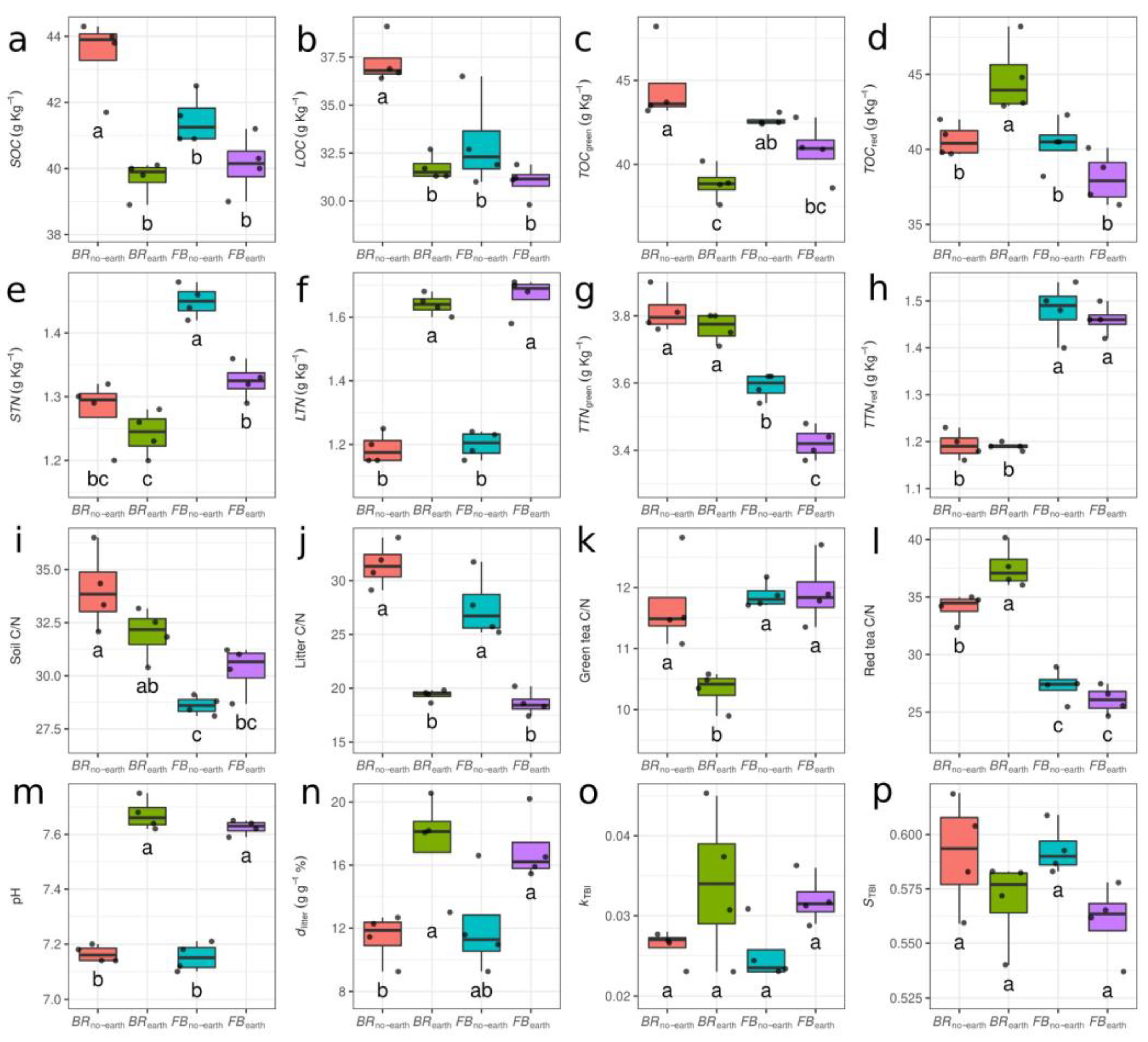
Figure 3.
α-diversity index (evenness) for bacterial communities in soils under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). Statistical significance was determined using the Kruskal-Wallis test. The measures within each treatment are in triplicate.
Figure 3.
α-diversity index (evenness) for bacterial communities in soils under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). Statistical significance was determined using the Kruskal-Wallis test. The measures within each treatment are in triplicate.
Figure 4.
Principal coordinate analysis (PCoA) plots of β-diversity, calculated using the weighted UniFrac metric, for bacterial communities in soils under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). The proportion of the data variation is displayed as axis percentages. The measures within each treatment are in triplicate.
Figure 4.
Principal coordinate analysis (PCoA) plots of β-diversity, calculated using the weighted UniFrac metric, for bacterial communities in soils under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). The proportion of the data variation is displayed as axis percentages. The measures within each treatment are in triplicate.
Figure 5.
Distance of the group of treatments in (a) broccoli with earthworms (BRearth), (b) broccoli without earthworms (BRno-earth), (c) faba bean with earthworms (FBearth), and (d) faba bean without earthworms (FBno-earth) based on the analysis of similarities (ANOSIM). The estimated R value (= 0.99) proved dissimilarity between groups (p < 0.001). The measures within each treatment are in triplicate.
Figure 5.
Distance of the group of treatments in (a) broccoli with earthworms (BRearth), (b) broccoli without earthworms (BRno-earth), (c) faba bean with earthworms (FBearth), and (d) faba bean without earthworms (FBno-earth) based on the analysis of similarities (ANOSIM). The estimated R value (= 0.99) proved dissimilarity between groups (p < 0.001). The measures within each treatment are in triplicate.
Table 1.
Number and total weight of earthworms measured at the beginning and at the end of the trial in soils under different treatments, consisting of broccoli with earthworms (BRearth) and faba bean with earthworms (FBearth). Each value represents the mean (± SD) from four measurements (n = 4). Means followed by different letters within columns are significantly different at 5% probability level (Tukey's HSD test).
Table 1.
Number and total weight of earthworms measured at the beginning and at the end of the trial in soils under different treatments, consisting of broccoli with earthworms (BRearth) and faba bean with earthworms (FBearth). Each value represents the mean (± SD) from four measurements (n = 4). Means followed by different letters within columns are significantly different at 5% probability level (Tukey's HSD test).
| Date |
Treatment |
Earthworms
(number)
|
Earthworms
(g)
|
| 28 November |
BRearth
|
61 ± 6 b |
14.56 ± 2.04 b |
|
FBearth
|
58 ± 2 b |
14.47 ± 1.04 b |
| 31 March |
BRearth
|
101 ± 9 a |
31.24 ± 4.49 a |
|
FBearth
|
107 ± 17 a |
32.94 ± 3.99 a |
Table 2.
Maximum shoot height, shoot fresh weight, maximum root length and root fresh weight in plants under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). Each value represents the mean (± SD) from four measurements (n = 4). Means followed by different letters within columns are significantly different at 5% probability level (Tukey's HSD test).
Table 2.
Maximum shoot height, shoot fresh weight, maximum root length and root fresh weight in plants under different treatments, consisting of broccoli with earthworms (BRearth), broccoli without earthworms (BRno-earth), faba bean with earthworms (FBearth), and faba bean without earthworms (FBno-earth). Each value represents the mean (± SD) from four measurements (n = 4). Means followed by different letters within columns are significantly different at 5% probability level (Tukey's HSD test).
| Treatment |
Maximum shoot height
(cm)
|
Shoot fresh weight
(g)
|
Maximum root height
(cm)
|
Root fresh weight
(g FW)
|
|
BRearth
|
16.9 ± 1.9 c |
355.08 ± 76.89 a |
69.0 ± 3.9 a |
317.06 ± 29.13 a |
|
FBearth
|
63.4 ± 5.4 a |
393.12 ± 44.45 a |
37.2 ± 3.2 c |
192.00 ± 15.50 c |
|
BRno-earth
|
15.3 ± 0.7 c |
309.14 ± 45.40 ab |
55.2 ± 3.8 b |
270.08 ± 26.84 b |
|
FBno-earth
|
51.0 ± 5.1 b |
292.24 ± 34.84 b |
31.2 ± 2.1 d |
157.78 ± 23.21 d |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).