1. Introduction
Strawberry (
Fragaria × ananassa Duch.) is a perennial herbaceous fruit tree species belonging to the Rosaceae family, and it is an economically important crop species widely cultivated worldwide [
1]. According to the statistics of the Chinese Horticultural Society, the total planting area of strawberry plants in China is approximately 133,400 hectares; the total output is 2 million tons, which ranks first in the world in both total area and total output [
2]. High-quality strawberry seedlings are the basis for high yield, and the quality of seedlings directly affects the yield [
3]. At present, domestic strawberry seedlings are generally grown in the open field, and seedlings are generally grown in May-July and transplanted in mid-July to August. When growing strawberry seedlings and cultivating them in open fields, the light intensity often exceeds 1800 μmol·m
-2·s
-1, far exceeding the saturation light intensity of strawberry plants (approximately 600 μmol·m
-2·s
-1), which is likely to cause photoinhibition because of excess light energy [
4]. Under natural conditions, high light is often accompanied by high-temperature, and high-temperature can aggravate the degree of photoinhibition induced by high-light [
5].
Photoinhibition is a phenomenon in which the photosynthesis rate and photosynthesis efficiency are reduced when the light energy captured by the light-harvesting pigment proteins of green plants exceeds the amount used by the photosynthetic mechanism; this phenomenon is manifested mainly in a reduction of the net photosynthesis rate (P
n), photochemical efficiency and quantum efficiency. The main site of photoinhibition is photosystem II (PSII) [
6,
7]. Both P
+680 and
1O
2 produced by PSII after being inhibited by light are strong oxidants that can oxidize and disrupt nearby chlorophyll (Chl) and D1 proteins, causing permanent damage to photosynthetic organs and a decrease in photosynthesis, eventually leading to plant death [
8]. To resist high-temperature and high-light stress, plants have evolved a protective defense mechanism to avoid the effects of photoinhibition and reduce photooxidative damage, such as increasing the photosynthesis rate and the activity of the reactive oxygen species–scavenging system [
9].
The chemical name of chitosan (CTS) is poly-N-acetylglucosamine and is generally referred to as polyglucosamine. CTS is the product of the deacetylation of the natural polysaccharide chitin, which is quite abundant and ranks second in terms of the earth's renewable resources [
10]. Owing to its nontoxicity, biocompatibility, broad-spectrum antibacterial properties, and permeability-enhancing properties, CTS has been widely used in agriculture as a soil amendment, plant growth regulator and promoter of cold resistance [
11]. Studies have shown that CTS can improve the cold resistance of rice seedlings [
12] and promote rice seed germination and seedling growth under salt-stress conditions [
13]. Wang et al. [
14] pointed out that exogenous CTS can reduce the malondialdehyde (MDA) content; increase the contents of Chl, proline and soluble protein; enhance the activity of antioxidant enzymes; and alleviate the damage to cucumber seedlings under high-temperature stress. However, the role of CTS in the response to high-temperature stress and high-light stress combined has not been reported, and its effect on strawberry under high-temperature stress and high-light stress combined is still unclear.
In this study, Xuelixiang strawberry was used as a test material, and 100 mg·kg-1 CTS solution was sprayed onto the leaves. Relevant indicators such as strawberry Chl content, membrane peroxidation, reactive oxygen species content, antioxidant enzyme content and reduced ascorbic acid (AsA)-reduced glutathione (GSH) cycle activity were determined under the combined stress of high-temperature and high-light, and the role of exogenous CTS in the process of strawberry resistance to high-temperature and high-light stress was studied. The mechanism associated with the resulting physiological and biochemical effects provides a theoretical basis for reducing the damage caused by high-temperature and high-light stress in the production of strawberry seedlings.
2. Materials and Methods
2.1. Materials and Design
In this study, Xuelixiang strawberry was used as the test variety and cultivated in the Innovation Park of Shandong Agricultural University. Three-leaf, one-hearted seedlings were selected for treatment. The CTS treatment involved foliar spraying of a CTS (the concentration was 100 mg·kg-1; preliminary tests were conducted to select the optimum spraying concentration) solution, and the H2O treatment involved foliar spraying of deionized water. Each treatment included 28 strawberry seedlings and was replicated three times. The plants were sprayed once a day at 5:00 p.m. for three consecutive days, placed in an incubator (23°C and 400 μmol·m-2·s-1) for 12 h, and then subjected to a high-temperature and high-light stress treatment (38°C and 1800 μmol·m-2·s-1) (through preliminary experimental screenings, it was determined that the high- temperature stress and high-light stress consisted of 38°C + 1800 μmol·m-2·s-1). Serving as controls, plants were sprayed deionized water and grown under a suitable temperature and light (23°C+ 400 μmol·m-2·s-1). Fresh leaves of strawberry seedlings were taken at six time periods, namely, 0 h, 0.5 h, 1 h, 2 h, 4 h and 8 h, after treatment, frozen in liquid nitrogen, and stored at -80°C.
2.2. Determination Indicators and Methods
2.2.1. Chl Content Determination
Using the ethanol extraction method [
15], we extracted 0.2 g of strawberry leaves in 20 mL of 95% ethanol for 24 h. The absorbance at 665 and 649 nm was subsequently measured using a UV-2600 spectrophotometer (Daojin, Suzhou) and substituted into formulas to calculate the Chl content.
2.2.2. Determination of Gas Exchange Parameters and Chl Fluorescence Parameters
Using a CIRAS-3 portable photosynthetic system (PP-Systems, USA), we selected five similarly growing leaves to measure the Pn, intercellular carbon dioxide concentration (Ci), stomatal conductance (gs) and transpiration rate (E). In addition, using an FMS-2 portable pulse-modulated fluorometer (Hansatech, UK), we selected five similarly growing leaves, and the initial fluorescence (Fo), maximum fluorescence (Fm), and maximum photochemical efficiency (Fv/Fm) were measured; the leaves were sufficiently allowed to adapt to the dark for 30 min prior to the measurements.
2.2.3. Tissue Staining and Determination of the Contents of H2O2 and O2-
Tissues were stained for H
2O
2 and O
2- using 1% 3,3’-diaminobenzidine (DAB) and 0.5% nitro blue tetrazolium (NBT), respectively, according to the methods of Tao et al. [
16]. The H
2O
2 content was determined according to the methods of Lin et al. [
17]. We extracted 0.5 g of fresh leaves of strawberry seedlings were collected, to which acetone was added. The material was then ground into a slurry and centrifuged at 3000 r/min for 10 min. Afterward 1 mL of supernatant was removed, and 0.1 mL of 5% titanium sulfate and 0.2 mL of concentrated ammonia in H
2O were added. The material was subsequently centrifuged at 3000 r/min for 10 min. After the precipitate formed, the supernatant was discarded. The plant pigments were then removed with acetone, after which 5 mL of 2 mol/L sulfuric acid and 10 mL of deionized H
2O were added. The absorption value of the reaction solution at a wavelength of 415 nm was then measured, and the corresponding H
2O
2 content was calculated according to a known H
2O
2 curve. A kit (Geruisi, Suzhou) was used to measure the O
2- content.
2.2.4. Determination of the Proline Content
The proline content was determined by the acid ninhydrin method [
18]. We extracted 0.5 g together with 5 mL of 3% sulfosalicylic acid were ground into a slurry. After extraction in boiling water for 10 min, and 2 mL of the supernatant was collected. Afterward, 2 mL of glacial acetic acid and 3 mL of 5% ninhydrin chromogenic solution were added, and 5 mL of toluene was added after the material was incubated in a 100°C water bath for 40 min. The supernatant was collected after layering, and the absorption value of the reaction solution was measured at a wavelength of 520 nm according to proline standards. The corresponding proline concentration was calculated according to the curve.
2.2.5. Determination of the Relative Conductivity
The relative conductivity was determined according to the methods of Zhang [
19]. We extracted 0.5 g of fresh leaves were obtained and then immersed in deionized water for 12 h. R1 was subsequently measured with a conductivity meter, and R2 was measured after 30 min in boiling water. The relative conductivity was calculated as R1/R2·100%
2.2.6. Determination of the MDA Content
The MDA content was determined by the thiobarbituri
c acid method [
20]. After plant material was added to 10 mL of 50 mol/L phosphate buffer solution (pH=8; including 1% polyvinylpyrrolidone (PVP), 0.1 mM EDTA and 1% Triton X-100), the mixture was ground to a slurry in an ice bath and then centrifuged at at 12000 rpm at 4°C for 10 min, and the supernatant was collected. Then, 1.5 mL of enzyme solution was added to 2.5 mL of TCA-TBA reaction solution, which was then boiled in water for 15 min and centrifuged at 4800 rpm for 10 min after cooling. The absorbance was measured at wavelengths of 532 nm and 600 nm, after which the values were substituted into the formula to calculate the MDA content.
2.2.7. AsA and GSH Contents and Determination of the AsA/DHA GSH/GSSG Ratios
The contents of AsA, GSH, oxidized ascorbic acid (DHA) and oxidized glutathione (GSSG) were determined using kits (Geruisi, Suzhou).
2.2.8. Determination of AsA-GSH Cycle-related Enzyme Activities
The activities of ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR) and dechlorascorbate reductase (DHAR) were determined using kits (Geruisi, Suzhou).
2.3. Statistics and Data Analysis
The means of three replications of the above indicators were determined and collated using Excel. SPSS 24 was used to perform univariate significant ANOVA, and Prism was used to construct the charts.
3. Results
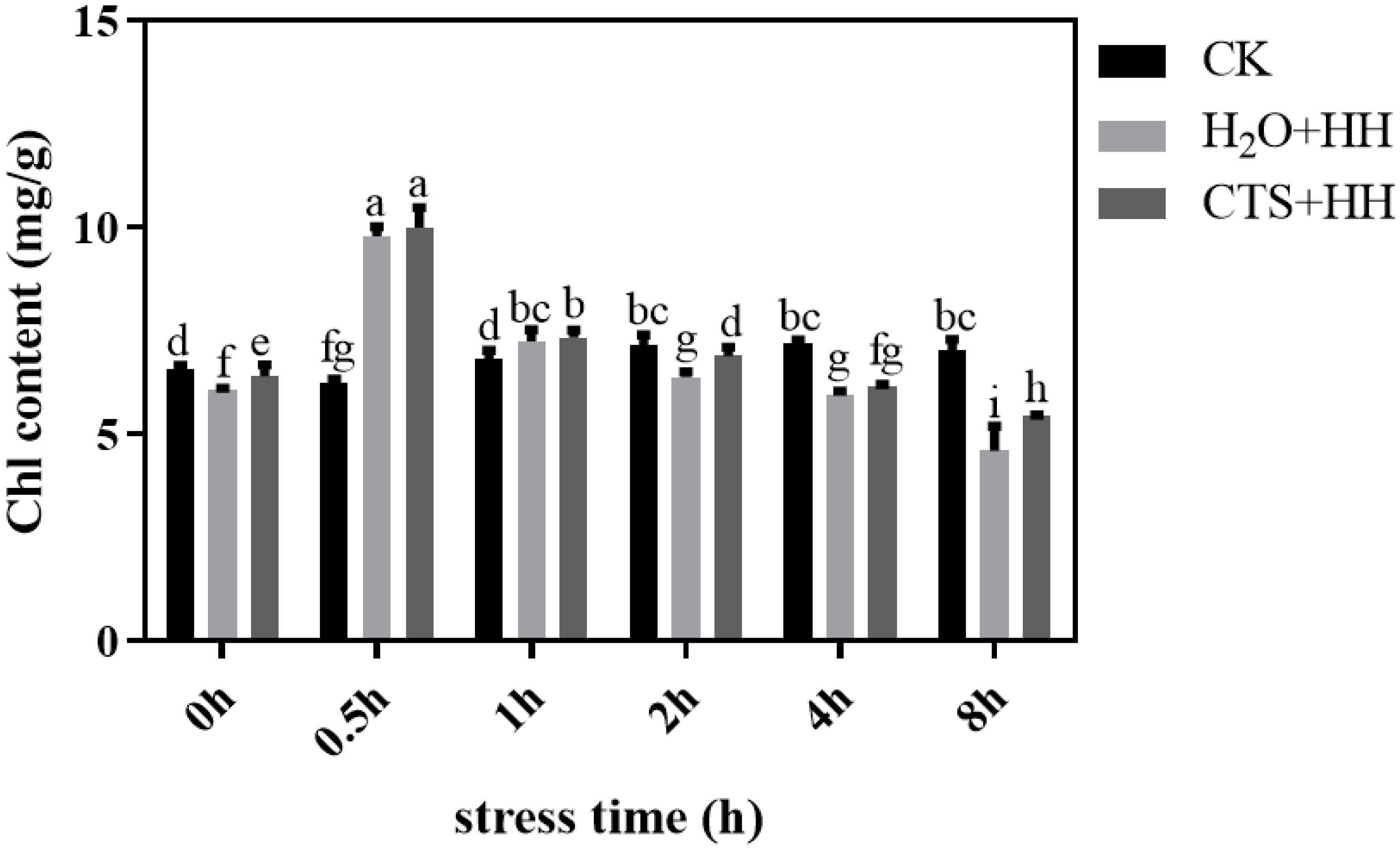
3.1. Effect of CTS on Chl Content
As shown in
Figure 1, with increased duration of high-temperature and high-light stress, the Chl content first increased and then decreased. The Chl content increased sharply after 0.5 h of stress treatment but decreased slowly after 1-8 h, and the Chl content under the CTS treatment was higher than that under the H
2O treatment. The longer the stress duration was, the more significant the difference was.
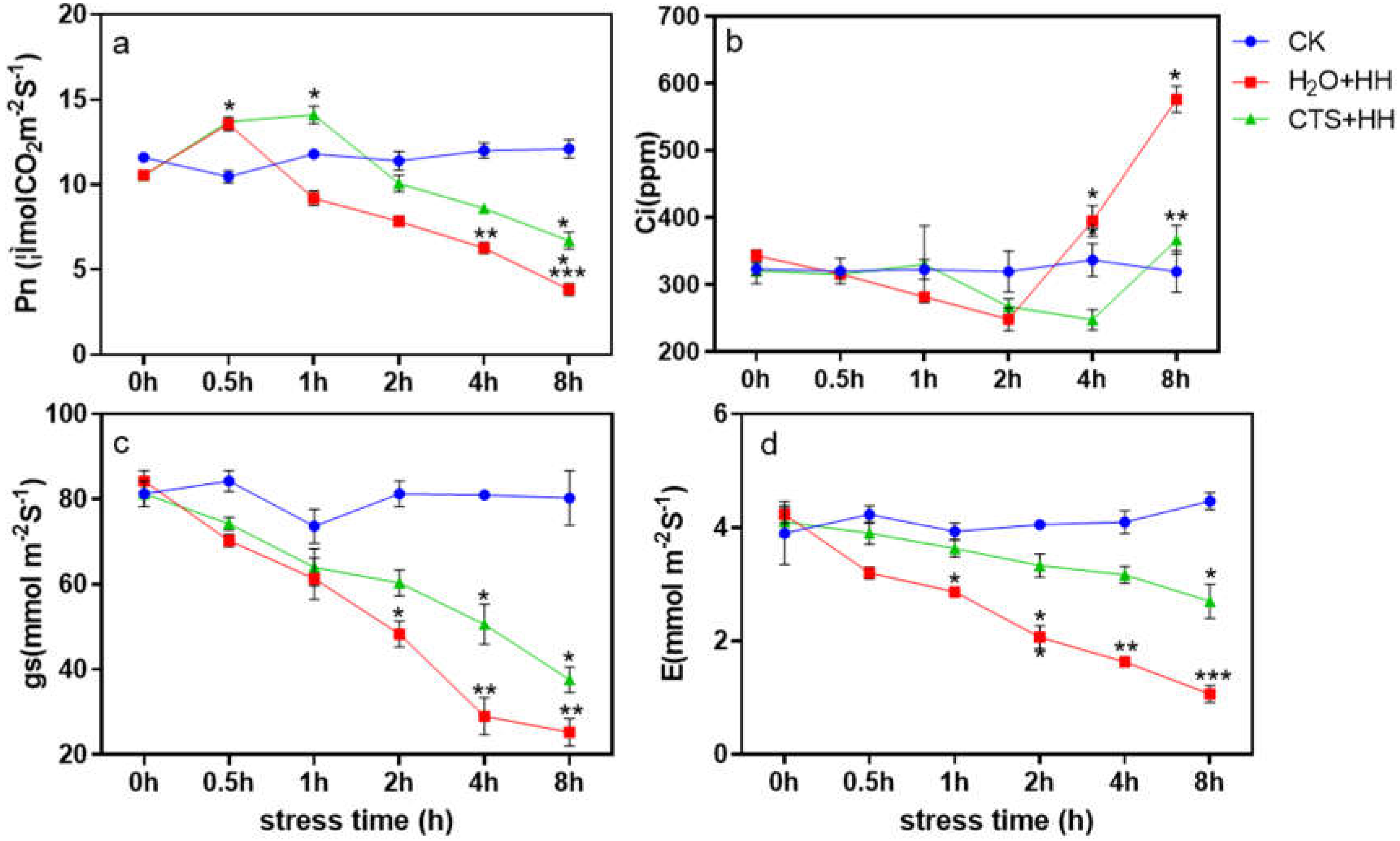
3.2. Effect of CTS Treatment on Gas Exchange Parameters
After high-temperature and high-light stress, the P
n of each treatment first increased and then decreased. At 8 h, the CTS treatment and H
2O treatment decreased by 39.4% and 66.2%, respectively. (
Figure 2a); 0-2 h after stress, C
i decreased slowly in the H
2O treatment and rose sharply from 2-8 h. After stress, C
i in the CTS treatment first decreased and then increased, and the rise was significantly slower than that in the H
2O treatment (
Figure 2b); g
s was similar to the change trend of E, it showed a downward trend with the prolongation of stress time, and the downward trend of CTS treatment was significantly slower than that of H
2O treatment (
Figure 2c and d).
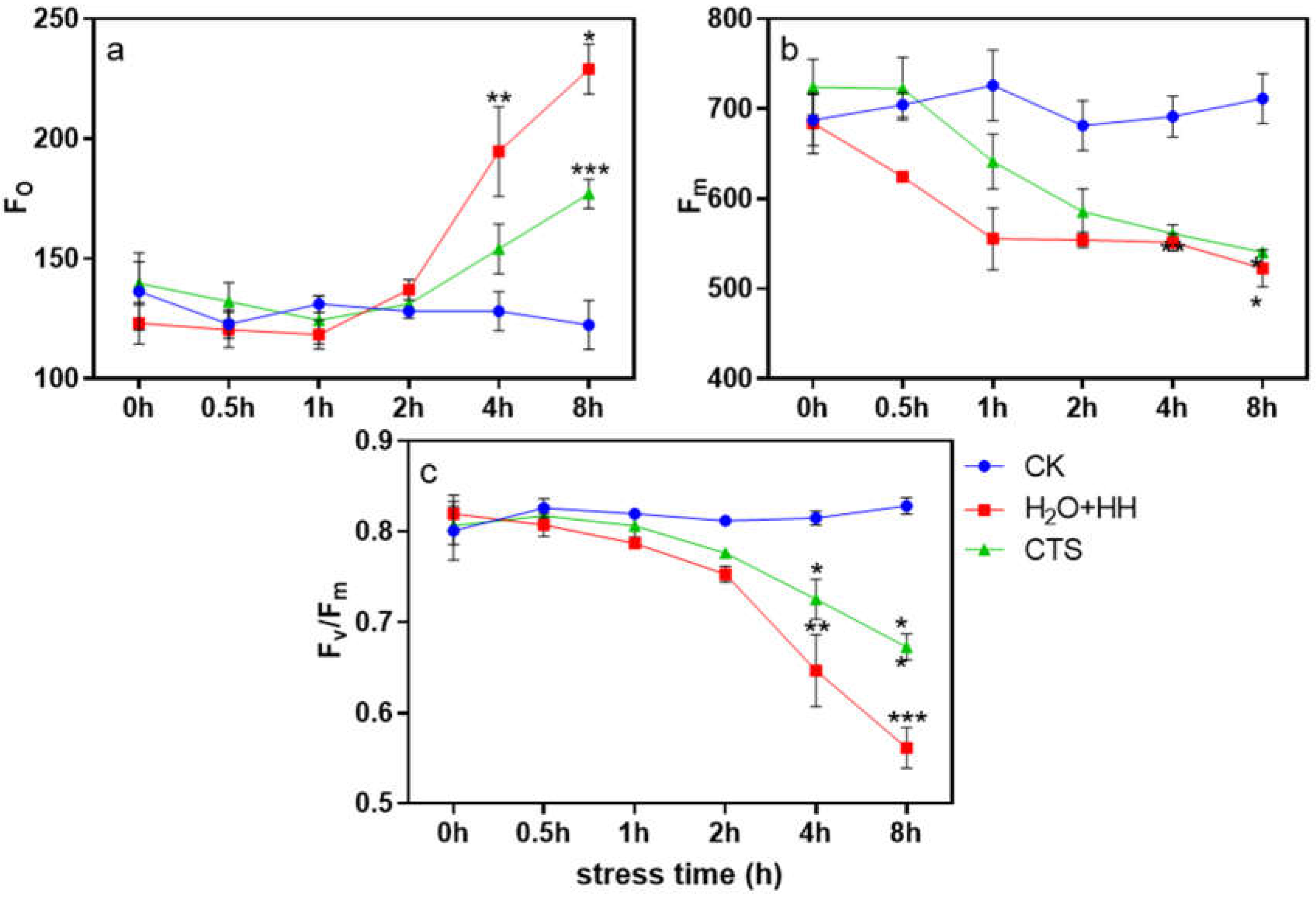
3.3. Effect of CTS on PSII Photoinhibition
After high-temperature and high-light treatment for 0-2 h, the F
o under each treatment was not significantly different; during 2-8 h stress, the F
o under the H
2O treatment and CTS treatment increased significantly, and the rising trend under the H
2O treatment was greater than that under the CTS treatment (
Figure 3a). The values of F
m and F
v/F
m decreased with increasing stress duration; when the stress was imposed for 8 h, the F
v/F
m value under the CTS treatment and H
2O treatment decreased by 18.8% and 32.3%, respectively, compared with that of the control (
Figure 3b and c).
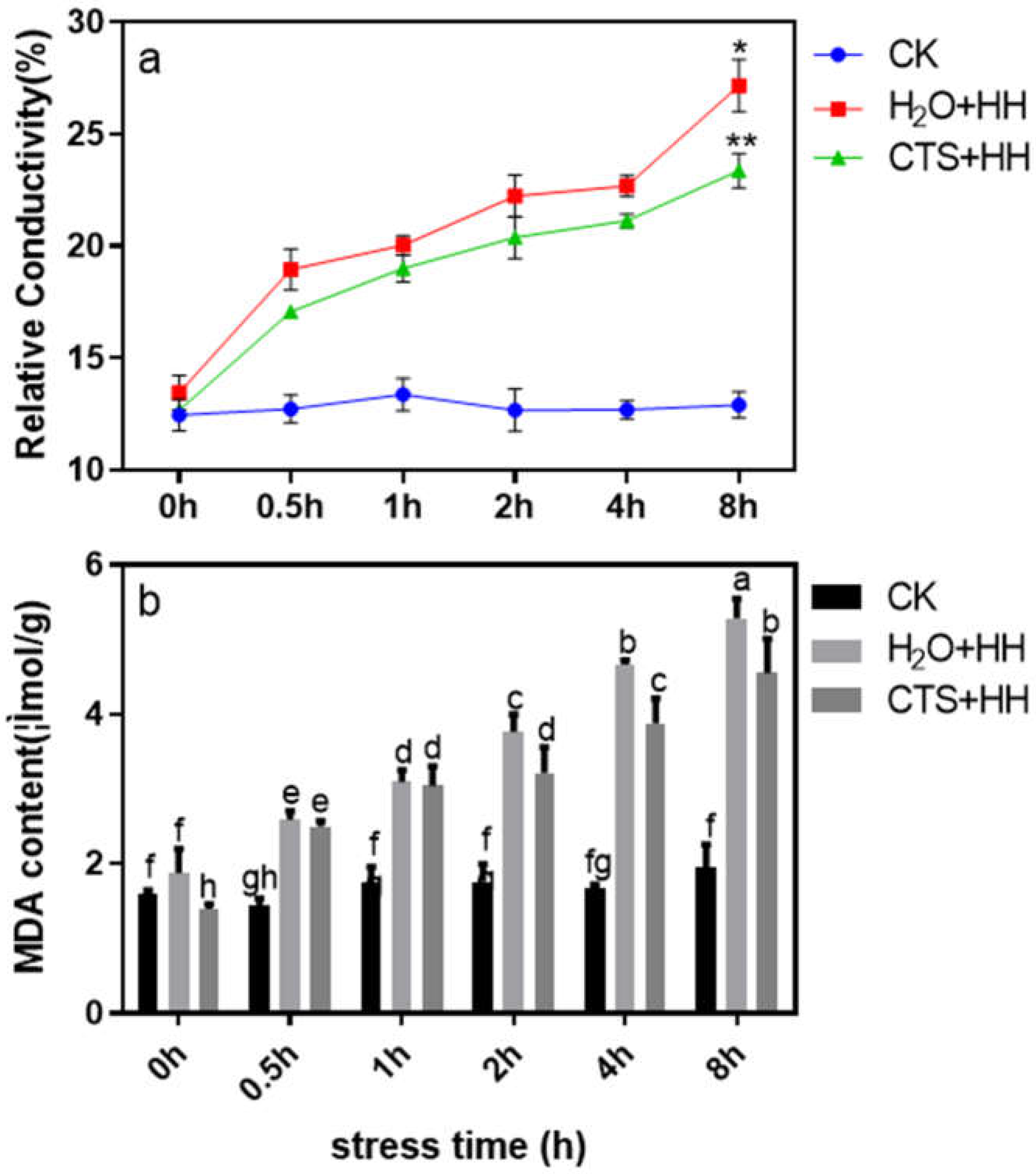
3.4. Effect of CTS on Membrane Peroxidation Level
Figure 4 shows that the MDA content in the strawberry leaves showed a significant upward trend after high-temperature and high-light treatment for 0-8 h, and the rate of upward under the CTS treatment was slower than that under the H
2O treatment. Compared with that under the control treatment, the electrical conductivity of the strawberry leaves under the CTS and H
2O treatments increased significantly (by 79.7% and 105.7%, respectively) when the plants were subjected to high-temperature and high-light for 8 h (
Figure 4a).
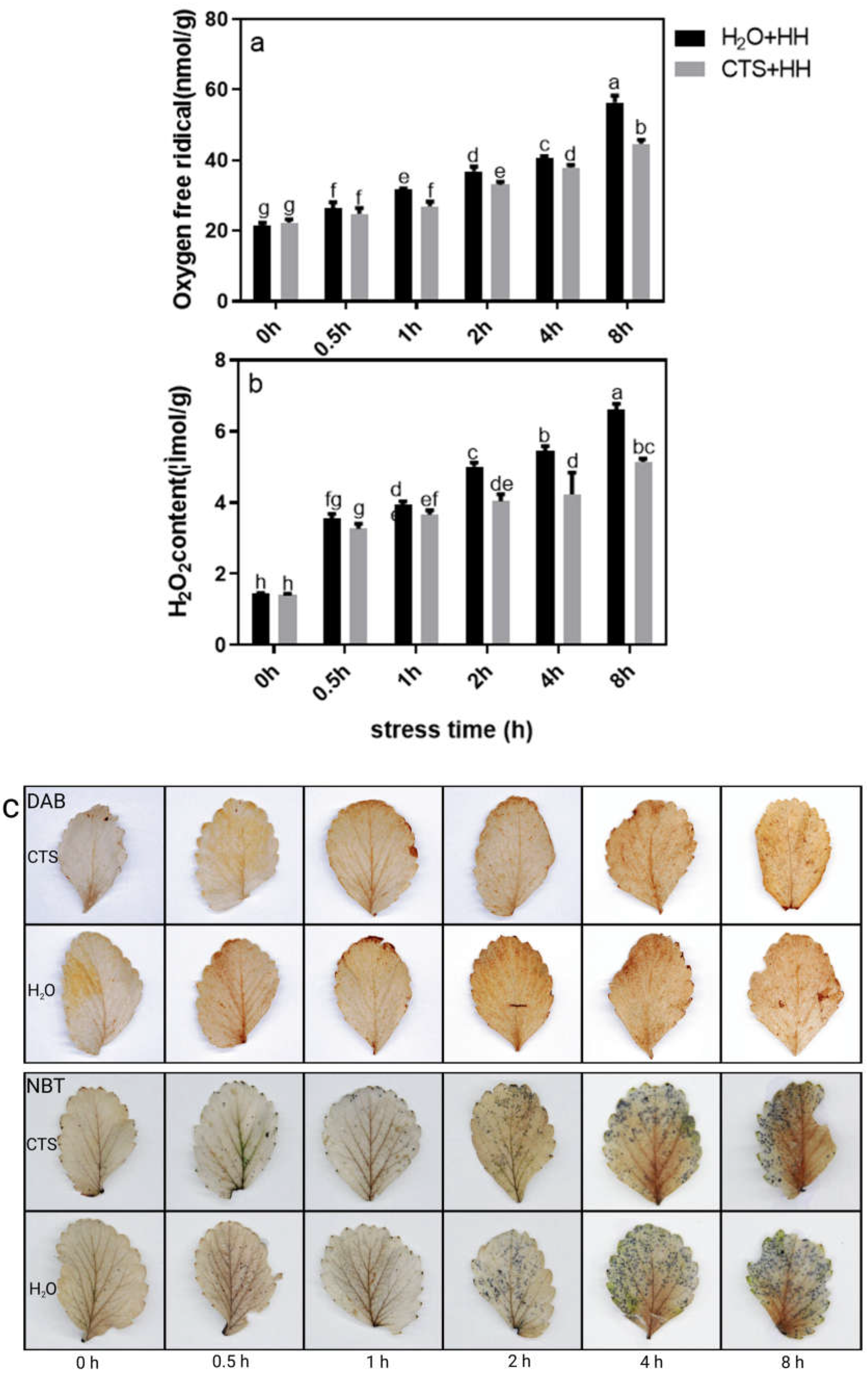
3.5. Effect of CTS on the H2O2 and O2- Contents
With increased stress duration, the H
2O
2 and O
2- contents in the strawberry leaves showed a significant upward trend, and the increasing trend of the H
2O
2 and O
2- contents under the H
2O treatment was significantly greater than that under the CTS treatment (
Figure 5a and b). The H
2O
2 and O
2- contents were consistent with the results of the DAB and NBT staining(
Figure 5c).
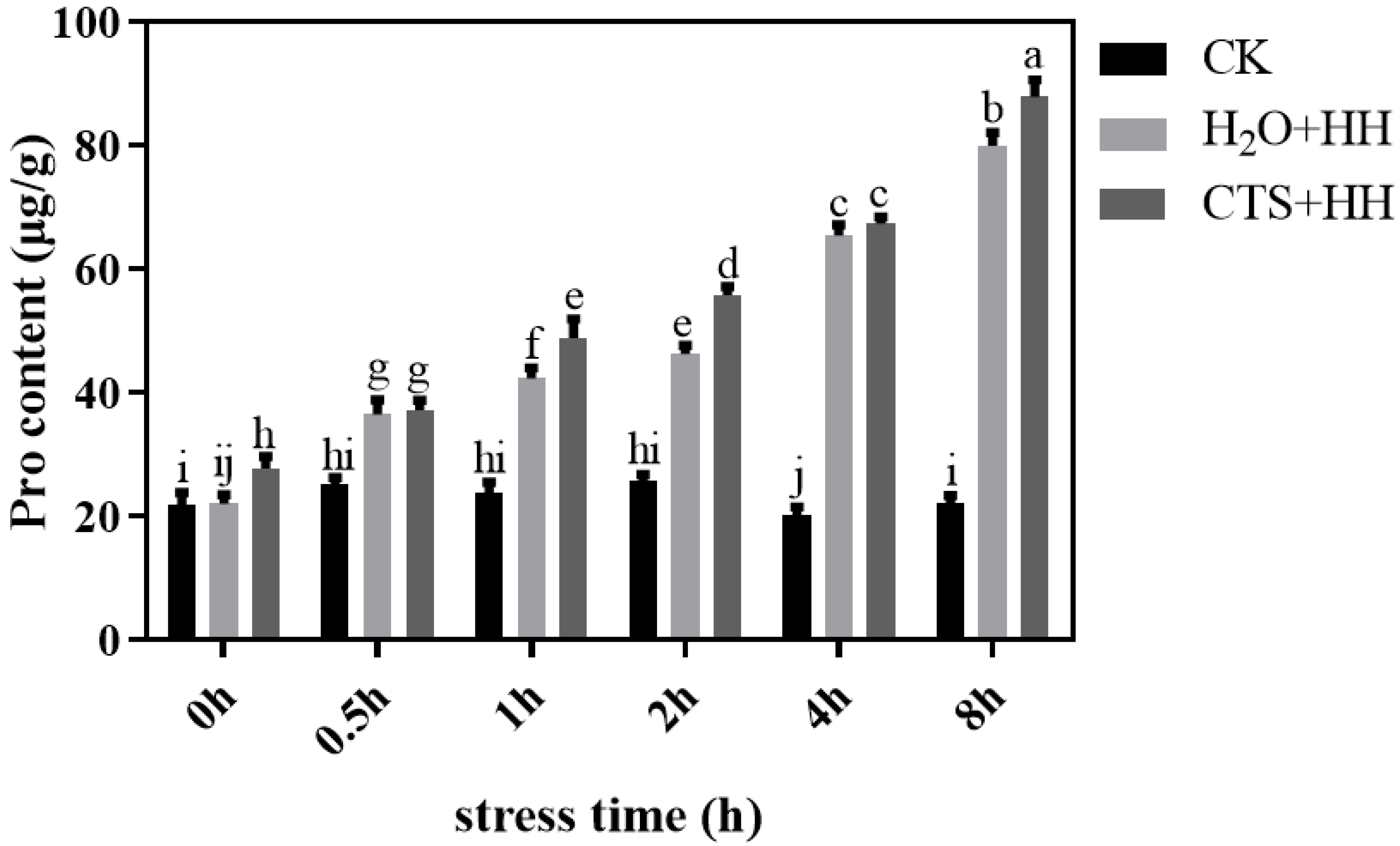
3.6. Effect of CTS on the Proline Content
High-temperature and high-light stress significantly led to an increase in proline content in the strawberry leaves. After 8 h of stress, the proline content under the CTS treatment and H
2O treatment increased by 130.1% and 99.9%, respectively, compared with that under the control treatment (
Figure 6).
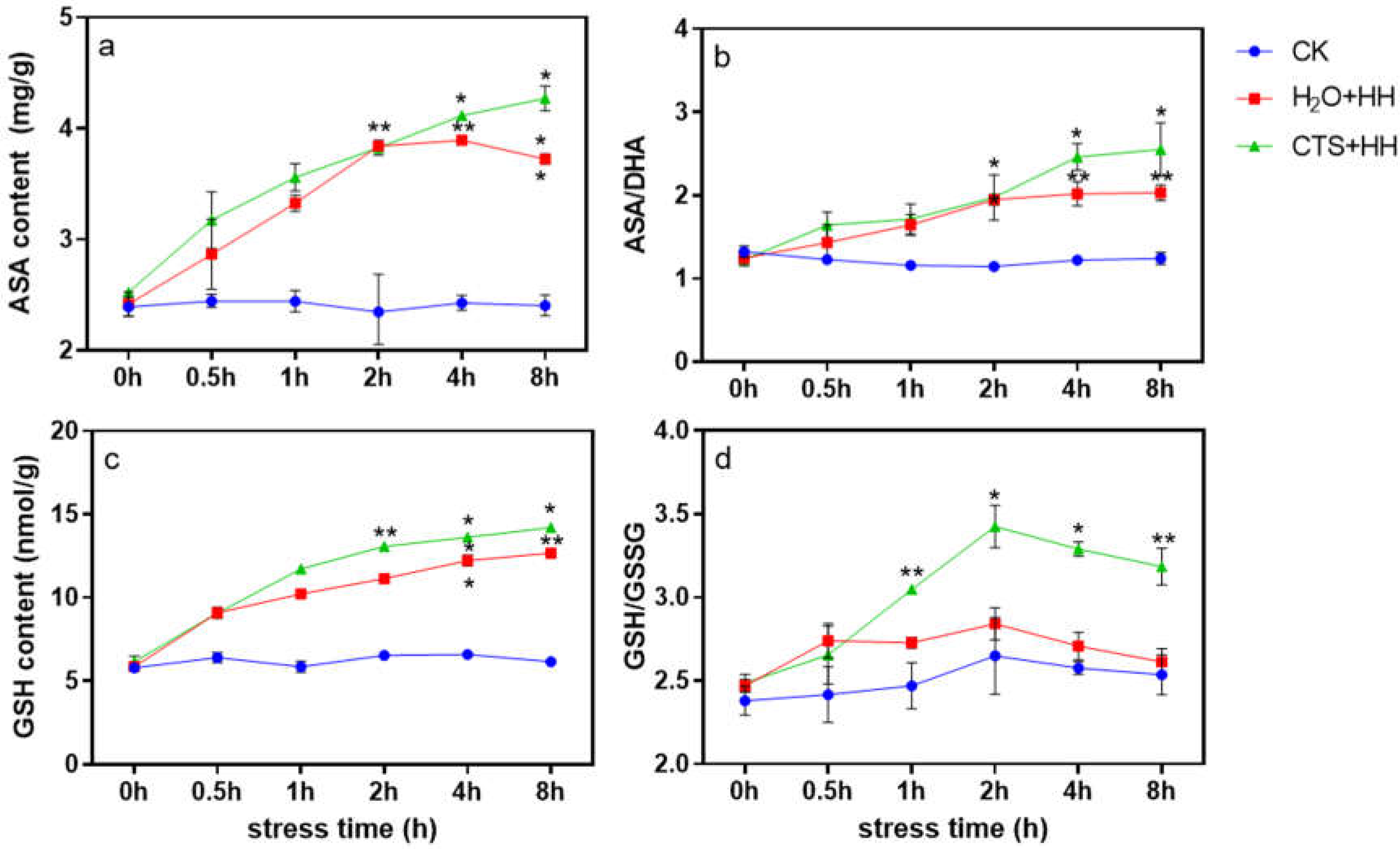
3.7. Effect of CTS on the AsA and GSH Contents and the AsA/DHA and GSH/GSSG Ratios
The AsA content under the H
2O treatment showed an upward trend within 2 h of high-temperature and high-light stress and a downward trend from 2 h to 8 h. The AsA content under the CTS treatment increased with prolonged stress time (
Figure 7a), and the GSH content under each treatment increased with prolonged stress time. According to the results, the GSH content under the CTS treatment was significantly higher than that under the H
2O treatment. After 8 h of stress, compared with that under the control treatment, the GSH content under the CTS treatment and H
2O treatment increased by 137.3% and 105.9%, respectively (
Figure 7c).
The AsA/DHA and GSH/GSSG ratios can be used as scales to judge the level of plant stress. As shown in
Figure 7b and d, within 2 h of stress, there was no significant difference in the AsA/DHA ratio between the CTS treatment and H
2O treatment. However, during 2 h to 8 h of stress, the AsA/DHA ratio under the CTS treatment was significantly higher than that under the H
2O treatment. Compared with the control, it increased by 25.6% (
Figure 7b). The GSH/GSSG ratio under the CTS treatment was significantly higher than that under the H
2O treatment (
Figure 7d).
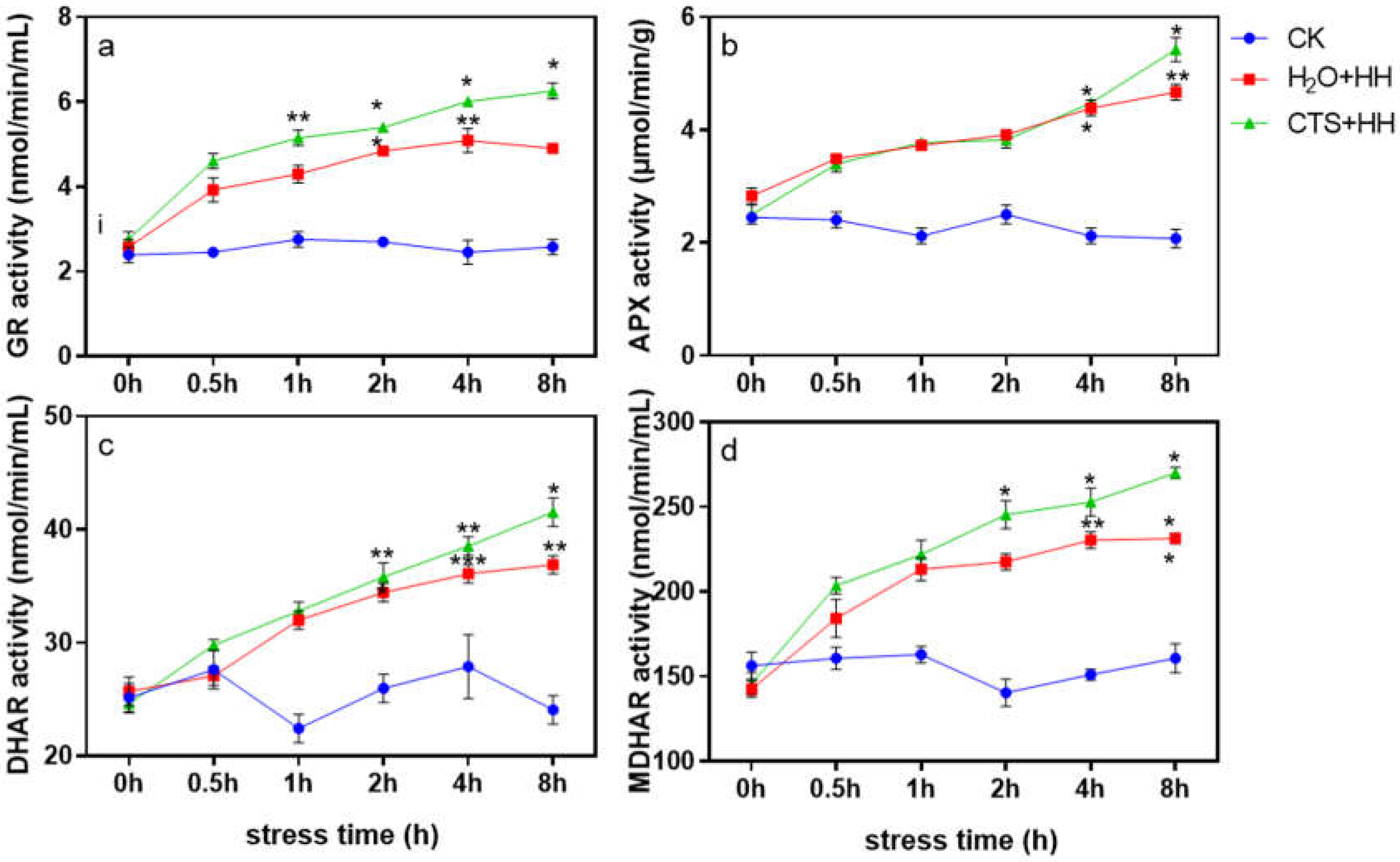
3.8. Effect of CTS on the Activities of key Enzymes of the AsA-GSH Cycle
As shown in
Figure 8, under the stress of high-temperature and high-light combined, the activities of GR, APX, DHAR and MDHAR under each treatment tended to increase with prolonged stress time. The GR, DHAR, and MDHAR activities in plants treated with CTS were significantly higher than those treated with H
2O, whereas the difference in APX activity was not significant (
Figure 8).
4. Discussion
In complex and dynamic natural environments, plants usually face a variety of stresses. The simultaneous occurrence of different stresses can lead to overlapping, potentiating or antagonistic effects [
21]. High-temperature and high-light are most common during the hot summer, and previous studies have shown that the simultaneous occurrence of hig-temperature and high-light can lead to the destruction of Chl, resulting in a decrease in Chl content [
22]. At the same time, too much light energy cannot be used quickly enough, and photosynthetic organs in turn can experience oxidative damage [
23]. The AsA-GSH cycle plays a very important role in maintaining redox balance [
24].
4.1. Effect of CTS on the Photosynthesis of Strawberry Seedlings Under High-temperature and High-light Stress
The Chl content is an important indicator of plant adaptations to environmental factors [
25]. Studies by Wei Xiaoling and others have shown that high-temperature and high-light stress can inhibit the synthesis of Chl in tobacco, wheat and other crops species and promote Chl degradation, thereby reducing the Chl content [
26,
27]. In addition, a decrease in Chl content can lead to decreased photosynthesis capacity and efficiency of plants [
28]. In this study, after 0-0.5 h of high-temperature and high-light stress, the Chl content under each treatment increased sharply (
Figure 1). This sudden increase in Chl content may be due to the sudden increase in light intensity and a sharp increase in plants to adapt to high amounts of photons. In addition, the Chl content decreased from 1-8 h after stress, and compared with with H
2O treatment, the CTS treatment significantly delayed the decline in Chl content (
Figure 1). Studies have shown that both high-temperature and high-light can reduce the photosynthesis rate [
29,
30]. In this study, the C
i under the H
2O treatment decreased significantly at 0-2 h of stress and increased sharply after 2 h; the C
i under the CTS treatment increased after 4 h, and but this increase was significantly less than that under the H
2O treatment (
Figure 2b). The reason for the increase in C
i was the destruction of cell structure and excess carbon dioxide, which led to an increase in the C
i [
23], and CTS can protect the cell structure to a certain extent. The P
n, g
s, and E of the plants in each treatment under high-temperature and high-light stress were lower than those under suitable temperature and light, although the CTS treatment delayed the decline in each index (
Figure 2a,c and d). This showed that CTS can alleviate the degradation of Chl caused by high-temperature and high-light stress, promote the synthesis of Chl, increase the content of Chl, and thus increase the photosynthesis rate of strawberry seedlings.
4.2. Effects of CTS on PSII Photoinhibition of Strawberry Seedlings Under High-Temperature and High-light Stress
Studies have shown that photoinhibition occurs when plants are stressed by both high-temperature and high-light [
5], and the main location of photoinhibition is PSII [
31]. F
o and F
m represent fluorescence yields when the PSII reaction centers are fully open and closed, respectively. The difference in the F
o under each treatment was statistically insignificant from 0-2 h after the high-temperature and high-light stress, and the F
o increased significantly during 2-8 h of the stress (
Figure 3a); deactivation of the PSII reaction center is the reason for the increase in F
o [
32]. CTS treated F
o increased the least, indicating that CTS can reduce the damage but cannot prevent it (
Figure 3a). The values of F
m and F
v/F
m decreased significantly with prolonged stress (
Figure 3b and c). However, spraying CTS maintained a certain level of F
v/F
m and reduced the degree of injury (
Figure 3c). This showed that spraying CTS can effectively alleviate the damage induced by high-temperature and high-light to PSII in strawberry leaves.
4.3. Effects of CTS on Reactive Oxygen Species Metabolism in Strawberry Seedlings Under High-temperature and High-light Stress
Stress can induce the production of reactive oxygen species in plants, and excessive reactive oxygen species have a strong toxic effect on cells [
33]. Studies have shown that CTS can reduce the H
2O
2 content in soybean under NaCl stress [
34] and the H
2O
2 and O
2- contents in corn under cadmium stress [
35]. Proline is an important osmotic adjustment substance in plants under stress. Plants can improve their stress resistance through increasing their proline content [
36]. In this study, with increasing high-temperature and high-light stress duration, the H
2O
2 and O
2- contents under each treatment showed an upward trend, and the H
2O
2 and O
2- contents under the CTS treatment were significantly lower than those under the H
2O treatment (
Figure 5a and b); however, the proline content was higher than that in the H
2O treatment (
Figure 6). These findings indicated that when strawberry plants are under stress, exogenous CTS can reduce the accumulation of reactive oxygen species, increase the content of osmotic adjustment substances, and thus improve the stress resistance.
4.4. Effects of CTS on Membrane Peroxidation of Strawberry Seedlings Under High-Temperature and High-light Stress
The integrity of the cell membrane system is crucial for plant growth and development. Stress resistance tests performed on various plant species have shown that the stability of the membrane system is closely related to the stress resistance of plants [
37,
38]. MDA is the final product of plasma membrane peroxidation, and changes in MDA content can reflect the degree of temperature stress on cell membrane damage [
39]. Tao et al. [
16] found that high-temperature and high-light stress can cause the MDA content in tomato plants to increase . It has also been shown that CTS can reduce the MDA content in walnut under cold stress [
40]. In this study, the MDA content in strawberry seedlings showed an increasing trend after the seedlings were subjected to high-temperature and high-light stress (
Figure 4b), which occurred because high-temperature and high-light disrupted the metabolic balance of reactive oxygen species, resulting in the accumulation of MDA; moreover, the relative electroconductivity increased with increasing stress duration (
Figure 4a). This showed that high-temperature and high-light disrupted the integrity of the membrane system of the strawberry seedlings, resulting in a large amount of electrolyte leakage. As the stress duration of the strawberry seedlings increased, the damage to the membrane system became more severe (
Figure 4a). After spraying CTS, we found that the increases in MDA and relative electroconductivity in the leaves of the stressed strawberry seedlings decreased (
Figure 4a and b), which indicated that CTS had a protective effect on the cell membrane system.
4.5. Effects of CTS on the AsA-GSH Cycle in Strawberry Seedlings Under High-Temperature High-light Stress
The AsA-GSH cycle plays an important role in the process of removing reactive oxygen species [
41]. This cycle can effectively remove the H
2O
2 produced during metabolism, and the substances and enzymes participating in the cycle interact and coregulate [
42]. As an important antioxidant enzyme in the AsA-GSH cycle, APX catalyzes the oxidation of AsA to scavenge reactive oxygen components such as O
2- and H
2O
2 [
43]; the regeneration reaction provides the reaction substrate for APX [
44]. AsA and GSH are important antioxidants in plant cells. The ratio of oxidative and reducing components (AsA/DHA, GSH/GSSG) is an important indicator of the redox state of cells. The content of AsA and GSH in cells and the AsA/DHA and GSH/GSSG ratios can accurately reflect the redox state of cells. In this study, under the combined stress of high-temperature and high-light, the AsA and GSH contents under the CTS treatment were significantly higher than those under the H
2O treatment (
Figure 7a and c), and the activities of the four key enzymes of the AsA-GSH cycle, namely, APX, GR, DHAR and MDHAR, increased after CTS was sprayed onto the plants (
Figure 8). The AsA and GSH contents and the AsA/DHA and GSH/GSSG ratio under the CTS treatment were also significantly higher than those under the H
2O treatment (
Figure 7), which showed that CTS can increase the contents of antioxidants in strawberry plants and the activity of key enzymes in the AsA-GSH cycle, thereby improving the stress resistance of strawberry.
5. Conclusion
In this study, the reactive oxygen species in strawberry seedling accumulated substantially under high-temperature and high-light stress, which coincided with damaged cell membranes, increased MDA contents, and decreased Chl contents. Spraying CTS onto strawberry leaves can increase antioxidant contents, osmoregulatory substances and antioxidant enzyme activity; quickly remove reactive oxygen species; reduce the damage of reactive oxygen species to photosynthetic mechanisms and cell membranes; and improve the photosynthesis efficiency of strawberry to reduce the damaging effects of high-temperature and high-light on strawberry.
Author Contributions
Conceptualization, E.F. ,L.L. and X.C.; methodology, E.F., H.L., X.W., W.X. and H.Z.; data curation, E.F.; writing—original draft preparation, E.F.; writing—review and editing, L.L.and Y.Z.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tan, C.; Dai, H.; Lei, J. World strawberry production and trade status and development trend. World Agric. 2003, 05, 10–12+40. [Google Scholar] [CrossRef]
- Gong, X.X. Research on Key Technologies of Strawberry Intensive Seedling Cultivation in High Temperature Season. Anhui Institute of Science and Technology, Anhui, 2016. [Google Scholar]
- Chen, W.Y. Key techniques for raising strawberry stolon seedlings. Northwest Horticulture (Comprehensive) 2021, 23–24. [Google Scholar]
- Xu, K.; et al. Response and mechanism of strawberry leaf photosynthesis to strong light. J. Appl. Ecol. 2005, 16, 73–78. [Google Scholar]
- Ming, W.; et al. Effect of spraying kaolin on the photosynthetic characteristics of Forg grape leaves under high temperature and strong light stress. Chin. Fruit, 2022; 57–61+67. [Google Scholar] [CrossRef]
- Dong, X.X.; et al. Research progress on the mechanism of plant photosystem adaptation to high light. J. Plant Physiol. 2016, 52, 1725–1732. [Google Scholar] [CrossRef]
- Powles, S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant physiol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Gan, Y.G.; Lian, J. Study on the effect of extracting chlorophyll from leaves of Aurantia chinensis with various extracts. Trop. Agric. Sci. 2021, 41, 86–91. [Google Scholar]
- Qin, S.H.; et al. Effects of exogenous Ca2+ on morphological and photosynthetic characteristics and chlorophyll fluorescent parameters of squash seedlings under high temperature and strong light stress. J. Appl. Ecol. 2010, 21, 2830–2835. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.S.; et al. Application of chitin and chitosan in agriculture. Chin. Agron. Bull. 2013, 29, 170–174. [Google Scholar] [CrossRef]
- Zhu, Y.L.; et al. Effect of chitosan on cold resistance of rice seedlings. Jiangsu Agric. Sci. 2017, 45, 66–68. [Google Scholar] [CrossRef]
- He, H.; et al. Effects of chitosan priming on rice seed germination and seedling growth under salt stress. J. Jiangxi Agric. Univ. 2022, 44, 1066–1074. [Google Scholar]
- Wang, T.; et al. Effects of exogenous chitosan on the growth of cucumber seedlings under high temperature stress. Jiangsu Agric. Sci. 2019, 47, 142–146. [Google Scholar] [CrossRef]
- Endang, K.; Ramadhani, A.N.; Putri, N.G.A.; Damara, V.C.J. Chlorophyll extraction methods review and chlorophyll stability of katuk leaves (Sauropus androgynous). J. Phys. Conf. Ser. 2021, 1858, 012015. [Google Scholar] [CrossRef]
- Tao, L.U.; Lu, J.; Qi, M.; Sun, Z.; Liu, Y.; Li, T. Protective roles of D1 protein turnover and the xanthophyll cycle in tomato under sub-high temperature and high light stress. Front. Agric. Sci. Eng. 2021, 8, 262–279. [Google Scholar] [CrossRef]
- Lin, Z.F.; et al. Relationship between accumulation of H2O2 in senescent leaves and chloroplasts and membrane lipid peroxidation. J. Plant Physiol. 1988, 01, 16–22. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, 2000. [Google Scholar]
- Zhang, R.; Xu, C.; Bao, Z.; Xiao, R.; Chen, X.; Xiao, W.; Li, D.; Fu, X.; Yang, C.; Li, L. Auxin alters sodium ion accumulation and nutrient accumulation by playing protective role in salinity challenged strawberry. Plant Physiol. Biochem. 2021, 164, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. The Mitigation Effect of Exogenous Selenium on Strawberry Seedlings under Low Temperature Stress and Its Effect on AsA-GSH cycle. Zhejiang University, Zhejiang, 2016. [Google Scholar]
- Faik, A.; Popova, A.; Velitchkova, M. Effects of long-term action of high temperature and high light on the activity and energy interaction of both photosystems in tomato plants. Photosynthetica 2016, 54, 611–619. [Google Scholar] [CrossRef]
- Zhang, S.B; Deng, Q.; Hao, Y. Effects of high temperature and high light on photosystem II activity in leaves of two Bauhinia species. Photosynthetica 2019, 57, 1094–1099. [Google Scholar] [CrossRef]
- Gang, S.; Liu, Y.F.; Lu, T.; Qi, M.F.; Guan, X.X.; Liu, Y.; Li, T.L. The effects of abscisic acid on photosynthesis in tomato under sub-high temperature and high light stress. J. Comput. Theor. Nanosci. 2016, 13, 7189–7198. [Google Scholar] [CrossRef]
- Chen, F.; et al. Effects of exogenous methyl jasmonate on the AsA-GSH cycle of maize seedlings under salt stress. Biol. Bull. 2021, 56, 44–48. [Google Scholar]
- Yue, Y.; Luo, X. Effects of high temperature stress on cotton chlorophyll. Agric. Technol. 2022, 42, 34–37. [Google Scholar] [CrossRef]
- Wei, X.L.; Feng, C.Q.; Huang, Y.X.; Xu, T.L.; Qiu, F.X.; Wu, Q.; Zheng, Y.J.; Li, W.Q.; He, H.Q. Magnesium alleviates high temperature and strong light on photosynthetic physiological damage in tobacco plants. Fujian Agric. J. 2022, 37, 33–41. [Google Scholar] [CrossRef]
- Ma, P.F.; Li, L.; Yang, Y.; Zhao, H.; Fu, X.; Zhang, C. Effects of salicylic acid on D1 protein phosphorylation and PS II performance in wheat leaf chloroplasts under high temperature and high light stress. J. Appl. Ecol. 2008, 19, 2632–2636. [Google Scholar]
- Zhao, H.Z.; et al. Major agronomic traits and photosynthetic properties of spatial environmentally mutagenic wheat chlorophyll deletion mutants. J. Crop 2011, 37, 119–126. [Google Scholar] [CrossRef]
- Xu, Y.F.; Fu, J.; Chu, X.; Sun, Y.; Zhou, H.; Hu, T. Nitric oxide mediates abscisic acid induced light-tolerance in leaves of tall fescue under high-light stress. Sci. Hortic. 2013, 162, 1–10. [Google Scholar] [CrossRef]
- Yu, Q.L.; Sun, W.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine improves the sucrose metabolism of lettuce to resist high-temperature stress. Plant Growth Regul. 2022, 96, 497–509. [Google Scholar] [CrossRef]
- Li, T.L.; et al. Mechanism of PSI of PS and photoinhibition in higher plants. J. Shenyang Agric. Univ. 2016, 47, 513–519. [Google Scholar] [CrossRef]
- Chen, M.M.; et al. Analysis of chlorophyll fluorescence under natural high temperature. Chin. Agric. Bull. 2022, 38, 86–95. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Y.; Zhang, W.W. Effect of exogenous chitosan on antioxidant system of vegetable soybean chloroplast under NaCl stress. J. Plant Nutr. Fertil. 2016, 22, 1356–1365. [Google Scholar] [CrossRef]
- Qu, D.Y.; et al. Effects of chitosan on the activities of antioxidant enzymes and endogenous hormones in maize seedling roots under cadmium stress. J. Northwest Bot. 2017, 37, 719–727. [Google Scholar] [CrossRef]
- Deng, T.; et al. Chitosan stimulates antifreeze response of walnut under low temperature stress. J. Northwest. For. Univ. 2016, 31, 60–64. [Google Scholar] [CrossRef]
- Wang, G.P. .; et al. The effect and mechanism of betaine on improving plant stress resistance. Jiangxi Agric. J. 2014, 26, 22–26. [Google Scholar] [CrossRef]
- Yan, D. Study on Physiological Indexes and Membrane Damage of Tomato Seedlings under Low Temperature Stress by Exogenous Substances. Northeast Agricultural University, 2007. [Google Scholar]
- Peng, C.C.; et al. Research progress on the relationship between biofilm and plant resistance. J. Hubei Univ. Nat. (Nat. Sci. Edn.) 1998, 6, 5–10. [Google Scholar]
- Duntuo; et al. Chitosan on walnut freezing response under cold stress. J. Northwest For. Coll. 2016, 31, 60–64. [Google Scholar] [CrossRef]
- Jing, Z.Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA enhances the antioxidant defense system of maize by regulating the AsA-GSH cycle under drought stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Lv, X.M.; Yang, Y.; Jin, J.; Bai, R. Effects of CaCl2 on the AsA-GSH cycle of sour jujube seedlings under NaCl stress. Acta Hortic. Sin. 2017, 44, 953–962. [Google Scholar] [CrossRef]
- Shaohua, L.; et al. Research progress on redox defense system of fruits and vegetables. Chin. Fruits Veg. 2021, 41, 33–39. [Google Scholar] [CrossRef]
- Qin, S.Y.; Liu, H.; Nie, Z.; Gao, W.; Li, C.; Lin, Y.; Zhao, P. AsA–GSH cycle and antioxidant enzymes play important roles in Cd tolerance of wheat. Bull. Environ. Contam. Toxicol. 2018, 101, 684–690. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).